Number Of Valence Electrons For Aluminum
listenit
Mar 18, 2025 · 5 min read
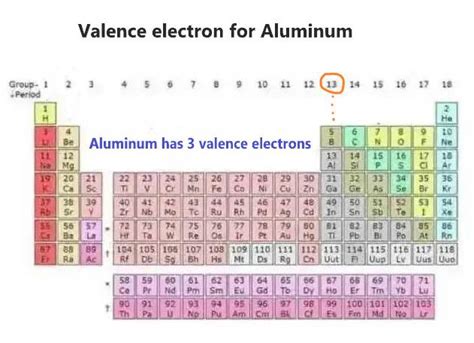
Table of Contents
Unveiling the Mysteries of Aluminum's Valence Electrons: A Deep Dive
Aluminum, a ubiquitous metal found in everything from soda cans to aircraft, holds a fascinating position in the periodic table. Understanding its properties, particularly its number of valence electrons, is key to comprehending its reactivity and diverse applications. This article delves deep into the world of aluminum's valence electrons, exploring its electronic configuration, bonding behavior, and the implications for its chemical and physical characteristics.
Understanding Valence Electrons: The Key to Reactivity
Before we focus specifically on aluminum, let's establish a foundational understanding of valence electrons. These are the electrons located in the outermost shell of an atom. They are crucial because they determine an element's chemical reactivity and how it will bond with other atoms. Atoms strive for stability, often achieved by having a full outermost electron shell. This drive for stability governs the formation of chemical bonds.
Atoms achieve stability through several mechanisms:
- Gaining electrons: Atoms with nearly full outer shells tend to gain electrons to complete their shell. This results in the formation of negatively charged ions (anions).
- Losing electrons: Atoms with few electrons in their outermost shell readily lose these electrons to achieve a stable, lower energy state. This forms positively charged ions (cations).
- Sharing electrons: Atoms can share electrons with each other to achieve a stable configuration. This leads to the formation of covalent bonds.
The number of valence electrons an atom possesses dictates which of these mechanisms it will most likely employ.
Aluminum's Electronic Configuration: A Closer Look
Aluminum (Al), with an atomic number of 13, possesses 13 electrons. To understand its valence electrons, we need to examine its electronic configuration. This describes how electrons are distributed among different energy levels (shells and subshells) within the atom.
The electronic configuration of aluminum is 1s²2s²2p⁶3s²3p¹.
This notation tells us:
- 1s²: Two electrons occupy the first energy level (shell) in the 1s subshell.
- 2s²: Two electrons occupy the second energy level in the 2s subshell.
- 2p⁶: Six electrons occupy the second energy level in the 2p subshell.
- 3s²: Two electrons occupy the third energy level in the 3s subshell.
- 3p¹: One electron occupies the third energy level in the 3p subshell.
The 3s²3p¹ electrons are the valence electrons. These are the outermost electrons, and they are the ones involved in chemical bonding. Therefore, aluminum has three valence electrons.
The Significance of Three Valence Electrons: Implications for Bonding
The presence of three valence electrons profoundly influences aluminum's chemical behavior. Aluminum is a metal, and metals generally lose electrons to form positive ions. With three valence electrons, aluminum readily loses these electrons to achieve a stable octet configuration, resembling that of the noble gas neon. This explains its +3 oxidation state, meaning it forms ions with a 3+ charge (Al³⁺).
The loss of three electrons is energetically favorable for aluminum, contributing to its high reactivity, especially with non-metals. This reactivity is evident in several key ways:
- Formation of ionic compounds: Aluminum readily reacts with non-metals such as oxygen, chlorine, and sulfur to form ionic compounds. For instance, aluminum oxide (Al₂O₃) forms when aluminum reacts with oxygen, and aluminum chloride (AlCl₃) forms when aluminum reacts with chlorine. In these compounds, aluminum exists as an Al³⁺ ion.
- Formation of metallic bonds: In its metallic state, aluminum atoms are held together by metallic bonds. These bonds arise from the delocalized valence electrons, which are shared among a "sea" of electrons, creating a strong, cohesive structure. This accounts for aluminum's characteristic metallic properties like malleability, ductility, and excellent conductivity of heat and electricity.
- Amphoteric nature: Aluminum exhibits amphoteric behavior, meaning it can react with both acids and bases. This dual reactivity stems from its ability to either lose its valence electrons (acting as a metal) or accept electron pairs (acting as a Lewis acid). This amphoteric nature further underscores the versatility of its three valence electrons.
Aluminum's Reactions: Illustrating Valence Electron Behavior
Let's explore some specific reactions of aluminum to further illustrate the role of its three valence electrons:
1. Reaction with Oxygen:
4Al(s) + 3O₂(g) → 2Al₂O₃(s)
Aluminum reacts vigorously with oxygen to form aluminum oxide, a protective layer that passivates the aluminum surface, preventing further oxidation. This reaction exemplifies the readiness of aluminum to lose its three valence electrons to oxygen, forming the Al³⁺ ion.
2. Reaction with Chlorine:
2Al(s) + 3Cl₂(g) → 2AlCl₃(s)
Aluminum reacts with chlorine to form aluminum chloride, again showcasing aluminum's tendency to lose its three valence electrons and achieve a stable configuration.
3. Reaction with Acids:
2Al(s) + 6HCl(aq) → 2AlCl₃(aq) + 3H₂(g)
Aluminum reacts with acids like hydrochloric acid, producing aluminum chloride and hydrogen gas. This reaction highlights the metal's ability to donate its valence electrons to the hydrogen ions (H⁺) in the acid.
4. Reaction with Bases:
2Al(s) + 2NaOH(aq) + 6H₂O(l) → 2Na + 3H₂(g)
The reaction with bases like sodium hydroxide demonstrates aluminum's amphoteric nature. Here, aluminum reacts with the hydroxide ions (OH⁻) to form a complex ion, [Al(OH)₄]⁻, and hydrogen gas. This reaction involves the acceptance of electron pairs, acting as a Lewis acid.
Applications Leveraging Aluminum's Valence Electrons
The properties stemming from aluminum's three valence electrons have led to a vast array of applications:
- Packaging: Aluminum's resistance to corrosion and its malleability make it ideal for packaging materials, such as cans and foil.
- Transportation: Its lightweight nature and strength make aluminum crucial in aircraft manufacturing and automotive parts.
- Construction: Aluminum's durability and corrosion resistance are used extensively in building materials, window frames, and roofing.
- Electrical wiring: Its excellent electrical conductivity makes it a vital component in electrical wiring and transmission lines.
- Cooking utensils: Aluminum's heat conductivity makes it suitable for cookware.
Conclusion: The Undeniable Influence of Three Valence Electrons
The three valence electrons of aluminum are fundamentally responsible for its remarkable properties and diverse applications. Their ability to be readily lost or shared dictates its reactivity, leading to the formation of strong bonds and contributing to its unique characteristics. From everyday objects to advanced technologies, the impact of aluminum's three valence electrons is undeniable and continues to shape our world. A deeper understanding of this fundamental aspect of aluminum's atomic structure opens a window into the fascinating world of materials science and chemical reactivity. Further research and innovation continue to explore and harness the potential of this versatile metal.
Latest Posts
Latest Posts
-
Why Are Lipids Insoluble In Water
Mar 19, 2025
-
How Do You Find Phenotypic Ratio
Mar 19, 2025
-
Greatest Common Factor Of 16 And 40
Mar 19, 2025
-
How Many Atoms Are In A Door Per Cubic Meter
Mar 19, 2025
-
1 In 7 As A Percentage
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons For Aluminum . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
