Number Of Orbitals In A 3s Sublevel
listenit
Mar 23, 2025 · 6 min read
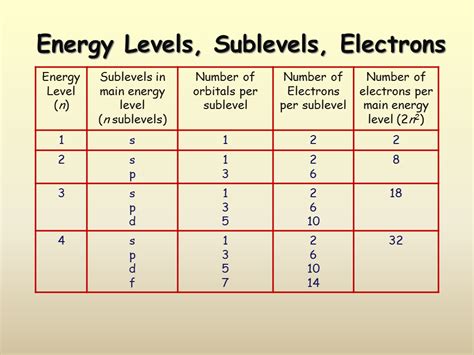
Table of Contents
Delving Deep: Understanding the Number of Orbitals in a 3s Sublevel
The seemingly simple question, "How many orbitals are in a 3s sublevel?" opens a door to a fascinating world of quantum mechanics and atomic structure. While the answer itself is straightforward, understanding the underlying principles requires exploring the nuances of electron configuration, quantum numbers, and the shapes of atomic orbitals. This article will delve deep into these concepts, providing a comprehensive explanation suitable for both beginners and those seeking a more thorough understanding.
Understanding Atomic Orbitals: A Quantum Mechanical Perspective
Before we tackle the 3s sublevel specifically, let's establish a foundational understanding of atomic orbitals. In the quantum mechanical model of the atom, an atomic orbital is a region of space around the nucleus where there's a high probability of finding an electron. Unlike the simplistic planetary model, orbitals don't define precise electron paths; instead, they describe probability distributions. This probabilistic nature is a crucial aspect of quantum mechanics, reflecting the inherent uncertainty in knowing both the position and momentum of an electron simultaneously (Heisenberg's Uncertainty Principle).
The characteristics of an atomic orbital are defined by a set of four quantum numbers:
-
Principal Quantum Number (n): This number determines the energy level and average distance of the electron from the nucleus. It's always a positive integer (n = 1, 2, 3,...). Higher values of 'n' indicate higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This number defines the shape of the orbital and its angular momentum. It can have integer values from 0 to n-1. For example, if n = 3, l can be 0, 1, or 2. These values correspond to different subshells: l = 0 (s subshell), l = 1 (p subshell), l = 2 (d subshell), and so on.
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. It can have integer values from -l to +l, including 0. For example, if l = 1 (p subshell), ml can be -1, 0, or +1, representing three p orbitals oriented along the x, y, and z axes, respectively.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum (spin) of the electron. It can have only two values: +1/2 (spin up) or -1/2 (spin down). This quantum number is crucial for understanding electron pairing within orbitals.
The 3s Sublevel: Unveiling its Structure
Now, let's focus on the 3s sublevel. The notation "3s" provides vital information:
-
3 (Principal Quantum Number): Indicates that the electrons in this sublevel reside in the third energy level (n = 3). This means they are further from the nucleus and possess higher energy than electrons in the 1s or 2s sublevels.
-
s (Azimuthal Quantum Number): Indicates that the sublevel has an azimuthal quantum number of l = 0. This signifies a spherical shape for the 3s orbital. The s subshell always contains only one orbital, regardless of the principal quantum number.
Why Only One Orbital in the 3s Sublevel?
The number of orbitals within a subshell is directly determined by the magnetic quantum number (ml). Since l = 0 for the s subshell, the only possible value for ml is also 0. This means there is only one possible orientation for the 3s orbital. Therefore, the 3s sublevel contains only one atomic orbital. This holds true for all s subshells (1s, 2s, 4s, etc.); they each possess a single spherical orbital.
Visualizing the 3s Orbital
Unlike the more complex shapes of p, d, and f orbitals, the 3s orbital is relatively simple to visualize. It's a sphere, but unlike the 1s orbital, the 3s orbital has regions of higher and lower electron probability density. It exhibits radial nodes – regions where the probability of finding an electron is zero. The 3s orbital has two radial nodes, showcasing the increasing complexity of orbitals as we move to higher energy levels. These nodes are spherical surfaces where the wave function changes sign.
Comparing 1s, 2s, and 3s Orbitals
It’s important to understand the difference between the 1s, 2s and 3s orbitals. While all are spherically shaped, their size and probability distributions differ. The 1s orbital is the smallest and closest to the nucleus. The 2s orbital is larger and has one radial node, and the 3s orbital is even larger with two radial nodes. This progression highlights the increase in energy and distance from the nucleus as the principal quantum number increases.
Implications for Electron Configuration and Chemical Bonding
The fact that the 3s sublevel contains only one orbital has significant consequences for the electron configuration of atoms and their chemical behavior. This single orbital can accommodate a maximum of two electrons, according to the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of four quantum numbers. Therefore, these two electrons must have opposite spins (one spin up, one spin down).
This limitation influences how atoms interact and form chemical bonds. The number of valence electrons – those in the outermost energy level – is crucial in determining an element's reactivity and bonding behavior. For elements where the 3s orbital is part of the valence shell, its capacity to hold only two electrons directly dictates the number of bonds that element can form.
Example: Sodium (Na)
Sodium (Na) has an atomic number of 11, meaning it has 11 electrons. Its electron configuration is 1s²2s²2p⁶3s¹. The single electron in the 3s orbital is its valence electron, making sodium highly reactive and readily losing this electron to achieve a stable noble gas configuration.
Beyond the Basics: Advanced Concepts
The discussion above provides a fundamental understanding. However, several more advanced concepts build upon these foundations:
-
Wave Function Representation: Atomic orbitals are mathematically described by wave functions (ψ), solutions to the Schrödinger equation. These wave functions provide a more precise description of the electron probability distribution within the orbital.
-
Radial and Angular Probability Distributions: Analyzing the radial and angular probability distributions helps visualize the three-dimensional shapes and probabilities of finding electrons at different distances and angles from the nucleus.
-
Hybridization: In molecular orbital theory, atomic orbitals can combine to form hybrid orbitals, changing their shapes and energy levels. This impacts the geometry and properties of molecules.
Conclusion: The Significance of the 3s Sublevel
Understanding the number of orbitals in the 3s sublevel is crucial for grasping the fundamental principles of atomic structure and quantum mechanics. The fact that it possesses only one orbital, capable of holding a maximum of two electrons, directly impacts electron configurations, chemical bonding, and the overall behavior of elements. While the concept might seem simple at first glance, its implications extend far beyond the basic level, illuminating the intricate world of atomic interactions and chemical reactivity. This knowledge forms the bedrock for understanding more complex chemical phenomena and the behaviour of matter at the atomic level.
Latest Posts
Latest Posts
-
What Element Is Always Present In An Organic Compound
Mar 25, 2025
-
What Is 25 As A Decimal
Mar 25, 2025
-
What Is The Decimal Of 4 6
Mar 25, 2025
-
How To Write Electron Configuration For Ions
Mar 25, 2025
-
How Many Feet Are In 0 4 Miles
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Number Of Orbitals In A 3s Sublevel . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
