Net Ionic Equation Of Hcl And Naoh
listenit
Mar 22, 2025 · 5 min read
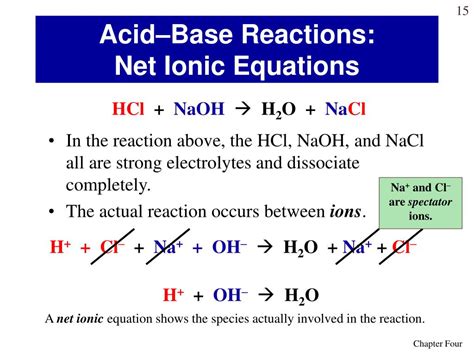
Table of Contents
Net Ionic Equation of HCl and NaOH: A Deep Dive into Acid-Base Reactions
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example of a strong acid-strong base neutralization reaction. Understanding this reaction, particularly its net ionic equation, is fundamental to grasping acid-base chemistry. This article will delve into the complete process, explaining the molecular, complete ionic, and net ionic equations, along with the underlying principles and applications.
Understanding the Reactants: HCl and NaOH
Before diving into the reaction itself, let's examine the properties of the individual reactants: hydrochloric acid (HCl) and sodium hydroxide (NaOH).
Hydrochloric Acid (HCl)
HCl is a strong acid, meaning it completely dissociates in water to form hydrogen ions (H⁺) and chloride ions (Cl⁻). This complete dissociation is key to understanding its behavior in reactions. The equation for the dissociation of HCl in water is:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
The "(aq)" denotes that the species are dissolved in aqueous solution (water).
Sodium Hydroxide (NaOH)
NaOH is a strong base, meaning it also completely dissociates in water, but into sodium ions (Na⁺) and hydroxide ions (OH⁻). Like HCl, its complete dissociation is crucial for understanding the reaction. The dissociation equation is:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
The Molecular Equation: Representing the Overall Reaction
The molecular equation shows the reactants and products in their undissociated forms. For the reaction between HCl and NaOH, the molecular equation is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This equation shows that hydrochloric acid reacts with sodium hydroxide to produce sodium chloride (table salt) and water.
The Complete Ionic Equation: Showing All Ions in Solution
The complete ionic equation takes the molecular equation a step further by representing all the soluble ionic compounds as their dissociated ions. Since both HCl and NaOH are strong electrolytes and completely dissociate, the complete ionic equation is:
H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
This equation shows all the ions present in the solution before and after the reaction occurs.
The Net Ionic Equation: Focusing on the Essential Reaction
The net ionic equation simplifies the complete ionic equation by removing spectator ions. Spectator ions are ions that appear on both sides of the complete ionic equation and thus do not participate directly in the reaction. In this case, Na⁺ and Cl⁻ are spectator ions. Removing them gives us the net ionic equation:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This equation represents the essence of the acid-base neutralization reaction: the hydrogen ions from the acid react with the hydroxide ions from the base to form water. This is the most important equation to understand as it highlights the core chemical change taking place.
Significance of the Net Ionic Equation
The net ionic equation offers several key advantages:
- Simplicity: It simplifies the representation of the reaction, focusing on the essential chemical changes.
- Generality: It applies to any strong acid-strong base neutralization reaction, regardless of the specific acid or base used. The net ionic equation will always be H⁺(aq) + OH⁻(aq) → H₂O(l).
- Predictability: It allows for the prediction of the products of similar reactions.
- Stoichiometry: It provides a clear stoichiometric relationship between the reactants and products, crucial for quantitative analysis.
Applications of the HCl and NaOH Reaction
The reaction between HCl and NaOH, and the understanding of its net ionic equation, has numerous applications across various fields:
-
Titration: This reaction is widely used in acid-base titrations to determine the concentration of an unknown acid or base. By carefully measuring the volume of HCl needed to neutralize a known volume of NaOH (or vice-versa), the concentration can be calculated. This is a fundamental technique in analytical chemistry.
-
pH Adjustment: The reaction can be used to adjust the pH of a solution. Adding HCl to a solution with excess OH⁻ ions will neutralize the base and lower the pH. Conversely, adding NaOH to a solution with excess H⁺ ions will neutralize the acid and raise the pH. This is crucial in many chemical processes where a specific pH is required.
-
Chemical Synthesis: The reaction can be a component of larger chemical syntheses, where the precise control of pH is essential for the formation of desired products.
-
Environmental Applications: In wastewater treatment, the reaction can be used to neutralize acidic or alkaline wastewater, making it safer for disposal or discharge into the environment.
Beyond Strong Acids and Bases: Weak Acids and Bases
The net ionic equation H⁺(aq) + OH⁻(aq) → H₂O(l) specifically applies to strong acid-strong base reactions. When dealing with weak acids or bases, the situation becomes more complex because these substances do not completely dissociate in water. For instance, the reaction between acetic acid (CH₃COOH, a weak acid) and NaOH would have a different net ionic equation because acetic acid only partially dissociates. The equilibrium nature of weak acid-base reactions needs to be considered when writing the net ionic equation.
Experimental Verification: Observing the Reaction
The reaction between HCl and NaOH can be easily demonstrated in a laboratory setting. Mixing solutions of HCl and NaOH will result in a noticeable temperature increase, indicating an exothermic reaction. Using a pH meter, one can observe the rapid change in pH as the neutralization occurs.
Conclusion: A Cornerstone of Chemistry
The net ionic equation for the reaction between HCl and NaOH, H⁺(aq) + OH⁻(aq) → H₂O(l), is a cornerstone concept in acid-base chemistry. Its simplicity and generality make it a valuable tool for understanding and predicting the behavior of strong acid-strong base reactions. Its applications span various fields, highlighting its significance in both theoretical and practical contexts. A deep understanding of this reaction and its representation through the net ionic equation is essential for anyone studying chemistry, from introductory courses to advanced research. This reaction serves as a springboard for learning more complex acid-base reactions and equilibrium concepts, emphasizing the interconnectedness of fundamental chemical principles. The ability to write and interpret net ionic equations is a critical skill for any chemist. Mastering this concept will strengthen your understanding of chemical reactions and their implications.
Latest Posts
Latest Posts
-
How Many Cups Are In Half Gallon
Mar 24, 2025
-
Heat Of Neutralization For Hcl And Naoh
Mar 24, 2025
-
What Type Of Bond Holds Amino Acids Together
Mar 24, 2025
-
Organism That Cannot Make Its Own Food
Mar 24, 2025
-
How Long Would It Take To Get To Andromeda
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Net Ionic Equation Of Hcl And Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
