What Type Of Bond Holds Amino Acids Together
listenit
Mar 24, 2025 · 6 min read
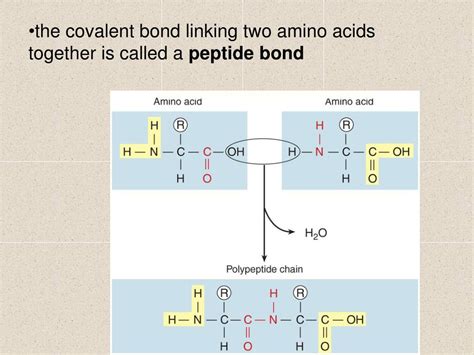
Table of Contents
What Type of Bond Holds Amino Acids Together? A Deep Dive into Peptide Bonds
The building blocks of life, proteins, are complex macromolecules with diverse functions. From catalyzing biochemical reactions (enzymes) to providing structural support (collagen), proteins underpin nearly all biological processes. But how do these intricate molecules form? The answer lies in the peptide bond, a special type of covalent bond that links amino acids together to create polypeptide chains, the precursors to proteins. This article will delve deep into the nature of the peptide bond, exploring its formation, properties, and significance in protein structure and function.
Understanding Amino Acids: The Building Blocks
Before exploring the peptide bond, let's briefly review the structure of amino acids, the monomers that form proteins. Each amino acid consists of a central carbon atom (the α-carbon) bonded to four groups:
- An amino group (-NH2): This group is basic and readily accepts a proton.
- A carboxyl group (-COOH): This group is acidic and readily donates a proton.
- A hydrogen atom (-H): This is a simple hydrogen atom.
- A side chain (R group): This is a variable group that differs among the 20 standard amino acids. The R group dictates the unique chemical properties of each amino acid – hydrophobic, hydrophilic, acidic, basic, etc. This variation in R groups is crucial for the vast diversity of protein structures and functions.
The specific sequence of amino acids in a polypeptide chain, known as the primary structure, determines the protein's final three-dimensional structure and ultimately its function.
The Peptide Bond: Formation and Characteristics
The peptide bond is a covalent bond formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another amino acid. This reaction, a condensation reaction or dehydration synthesis, releases a molecule of water (H2O). The bond that remains is the peptide bond, specifically an amide bond.
Formation:
The process involves several steps:
- Activation of the carboxyl group: The carboxyl group of one amino acid is activated, often through the addition of ATP (adenosine triphosphate), making it more reactive.
- Nucleophilic attack: The amino group of the second amino acid acts as a nucleophile, attacking the activated carboxyl carbon.
- Bond formation: A new covalent bond forms between the carbon atom of the carboxyl group and the nitrogen atom of the amino group.
- Water molecule release: A water molecule is released as a byproduct of the reaction.
The resulting bond is a peptide bond, represented as -CO-NH-. This bond is a key element that holds amino acids together in a linear chain.
Properties of the Peptide Bond:
The peptide bond possesses several unique characteristics that influence protein structure and function:
- Planarity: The peptide bond exhibits partial double-bond character due to resonance. This means the six atoms involved in the peptide bond (C, O, N, H, and the two α-carbons) lie in a plane. This planarity restricts rotation around the peptide bond, influencing the overall conformation of the protein.
- Polarity: The peptide bond is polar due to the electronegativity difference between the oxygen and nitrogen atoms. This polarity affects the hydrogen bonding interactions within the protein, playing a vital role in secondary and tertiary structures.
- Trans configuration: The peptide bond predominantly exists in the trans configuration, where the α-carbon atoms are on opposite sides of the peptide bond. This configuration minimizes steric hindrance between the side chains of adjacent amino acids.
- Rigidity: The partial double bond character contributes to the rigidity of the peptide bond. While rotation is restricted around the peptide bond itself, rotation is possible around the bonds adjacent to the α-carbon atoms (phi and psi angles), leading to the flexibility observed in protein structures.
Peptide Bond vs. Other Bonds in Proteins
While the peptide bond is the primary linkage connecting amino acids, other types of bonds contribute to the overall protein structure:
- Hydrogen bonds: These relatively weak bonds are critical for stabilizing secondary structures like alpha-helices and beta-sheets. They form between the carbonyl oxygen of one peptide bond and the amide hydrogen of another.
- Disulfide bonds: These strong covalent bonds form between the sulfur atoms of cysteine residues. They contribute to the tertiary structure and stability of some proteins.
- Hydrophobic interactions: These forces arise from the tendency of nonpolar side chains to cluster together, minimizing their contact with water. They are essential for protein folding and stability.
- Ionic bonds (salt bridges): These interactions occur between oppositely charged side chains of amino acids. They contribute to the tertiary and quaternary structures.
- Van der Waals forces: These are weak attractive forces between atoms in close proximity. While individually weak, their cumulative effect can be significant in protein folding.
Peptide Bond Cleavage: Hydrolysis
The peptide bond, while strong, is susceptible to hydrolysis, a process that breaks the bond by adding a water molecule. This process reverses the condensation reaction that formed the peptide bond. Hydrolysis can occur spontaneously under certain conditions or be catalyzed by enzymes known as peptidases or proteases. These enzymes play crucial roles in protein degradation and regulation.
Importance of Peptide Bonds in Protein Structure and Function
The peptide bond is fundamentally important for all aspects of protein structure and function:
- Primary structure: The sequence of amino acids linked by peptide bonds dictates the protein's primary structure, which sets the stage for higher-order structures.
- Secondary structure: The hydrogen bonding patterns involving the peptide bond's carbonyl and amide groups stabilize secondary structures, like alpha-helices and beta-sheets. These structures are formed due to local interactions within the polypeptide chain.
- Tertiary structure: The tertiary structure, the three-dimensional folding of a polypeptide chain, is determined by the interactions between amino acid side chains, including disulfide bonds, hydrophobic interactions, ionic bonds, and van der Waals forces. The peptide backbone, with its specific geometry defined by peptide bonds, provides a scaffold upon which the tertiary structure is built.
- Quaternary structure: Proteins with multiple polypeptide chains (subunits) have a quaternary structure. The peptide bonds within each subunit, and the non-covalent interactions between subunits, contribute to the overall quaternary structure and its function.
The precise arrangement of amino acids, connected by peptide bonds, determines the unique three-dimensional structure of each protein. This structure, in turn, directly influences the protein's function. Any alteration or damage to the peptide bonds can disrupt the protein's structure and lead to loss of function, potentially causing disease.
Peptide Bond Modifications: Post-translational Modifications
After a protein is synthesized (translation), its peptide bonds may undergo modifications. These post-translational modifications often regulate protein function or stability. Examples include:
- Isomerization: Changes in the cis-trans configuration of peptide bonds.
- Glycosylation: Attachment of sugar molecules to the amide nitrogen of asparagine residues.
- Phosphorylation: Addition of phosphate groups to serine, threonine, or tyrosine residues.
- Ubiquitination: Attachment of ubiquitin molecules, often targeting proteins for degradation.
These modifications demonstrate that peptide bonds, though crucial for the initial protein structure, aren't static and can be dynamically altered to fine-tune protein function.
Conclusion
The peptide bond, a seemingly simple covalent link between amino acids, is paramount to life. Its unique properties—planarity, polarity, and partial double-bond character—dictate the conformational possibilities of polypeptide chains, leading to the diverse structures and functions of proteins. Understanding the peptide bond is essential for grasping the complexities of protein structure, function, and their implications in health and disease. From the fundamental building blocks to the intricate folding patterns and post-translational modifications, the peptide bond remains the central player in the protein world, orchestrating the symphony of life.
Latest Posts
Latest Posts
-
What Percent Of 80 Is 35
Mar 26, 2025
-
How Many Different Combinations With 4 Numbers
Mar 26, 2025
-
What Is 13 16 As A Decimal
Mar 26, 2025
-
How Many Ounces Are In 3 4
Mar 26, 2025
-
Weight Of One Cubic Metre Of Water
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bond Holds Amino Acids Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
