Most Reactive Metal On The Periodic Table
listenit
Mar 28, 2025 · 6 min read
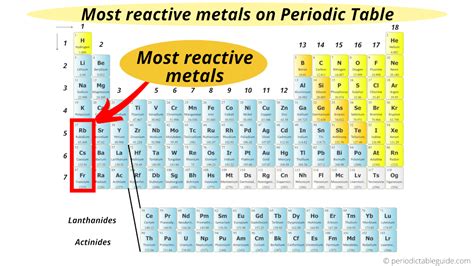
Table of Contents
The Most Reactive Metal on the Periodic Table: Understanding Francium's Extreme Reactivity
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Within this organized system, certain elements stand out for their extreme characteristics. One such characteristic is reactivity, and at the pinnacle of reactive metals sits francium (Fr), an element so rare and fleeting that its properties are largely theoretical. This article delves deep into the reasons behind francium's extreme reactivity, explores its unique characteristics, and compares it to other highly reactive alkali metals.
Understanding Reactivity: A Look at Electron Configuration
Before diving into francium's exceptional reactivity, let's establish a foundational understanding of what drives this property. Reactivity, in the context of metals, is primarily determined by the ease with which an atom can lose electrons to achieve a stable electron configuration. This stable configuration, often referred to as a noble gas configuration, is achieved when the outermost electron shell (valence shell) is completely filled.
Metals, by their very nature, tend to have relatively few electrons in their valence shell. They readily lose these electrons to attain a more stable state, forming positive ions (cations). The more readily a metal loses its electrons, the more reactive it is considered. This ease of electron loss is closely tied to factors like ionization energy and electronegativity.
-
Ionization Energy: This refers to the energy required to remove an electron from a neutral atom. Lower ionization energy indicates greater ease of electron loss and thus, higher reactivity.
-
Electronegativity: This measures an atom's tendency to attract electrons towards itself in a chemical bond. Metals generally have low electronegativity, meaning they are less likely to attract electrons and more likely to lose them.
Francium: The Most Reactive Metal
Francium, located in Group 1 (alkali metals) of the periodic table, possesses only one electron in its valence shell. This single electron is extremely loosely held, making it incredibly easy to lose. This contributes significantly to francium's exceptionally high reactivity. Several factors contribute to francium's extreme reactivity compared even to other alkali metals:
-
Low Ionization Energy: Francium boasts the lowest ionization energy of all elements. This means it requires the least amount of energy to remove its single valence electron, making it incredibly eager to participate in chemical reactions. This low ionization energy stems from the large atomic radius of francium; the valence electron is far from the positively charged nucleus and therefore experiences weaker electrostatic attraction.
-
Large Atomic Radius: As we move down Group 1, the atomic radius increases. This increase in atomic radius means the valence electron is further from the nucleus, resulting in weaker electrostatic attraction. Consequently, the electron is more easily lost. Francium, being at the bottom of Group 1, has the largest atomic radius among all alkali metals, resulting in the weakest attraction to its valence electron.
-
Shielding Effect: The inner electrons of an atom shield the valence electrons from the full positive charge of the nucleus. This shielding effect increases with the number of inner electrons. In francium, the numerous inner electrons effectively shield the valence electron from the nucleus, further reducing the electrostatic attraction and facilitating electron loss.
Comparing Francium to Other Alkali Metals
While other alkali metals like cesium and rubidium are also highly reactive, francium surpasses them all. Let's briefly compare francium's reactivity to some of its closest neighbors:
-
Cesium (Cs): Cesium, the element immediately above francium, is known for its extreme reactivity. It readily reacts with air and water, igniting spontaneously in air. However, francium's reactivity is significantly higher due to its larger atomic radius and lower ionization energy.
-
Rubidium (Rb): Rubidium, located above cesium, exhibits similar high reactivity to cesium, but again, francium's reactivity overshadows both.
-
Potassium (K), Sodium (Na), and Lithium (Li): As we move up Group 1, the reactivity decreases considerably. Potassium, sodium, and lithium are still reactive metals, but their reactivity is far less pronounced compared to francium, cesium, and rubidium. The differences are directly related to the increasing ionization energy and decreasing atomic radius as we ascend the group.
The Challenges of Studying Francium
Despite its position as the most reactive metal, studying francium presents significant challenges. Francium's extreme rarity and short half-life (around 22 minutes for its most stable isotope, Francium-223) severely limit research opportunities. It's not found naturally in significant quantities and is produced synthetically in tiny amounts, making extensive experimental analysis exceptionally difficult. The majority of our understanding of francium's properties is therefore based on theoretical calculations and extrapolations from its position within the periodic table and the trends observed in other alkali metals.
Reactivity in Practice: Reactions of Francium
Although direct experimental data on francium's reactivity is scarce due to its short half-life and rarity, theoretical predictions and limited experimental observations suggest the following:
-
Reaction with Water: Francium would react explosively with water, generating hydrogen gas and francium hydroxide. This reaction would be far more vigorous than even that of cesium, potentially resulting in a larger explosion due to the greater ease of electron loss.
-
Reaction with Air: Similar to other alkali metals, francium would react rapidly with oxygen in the air, forming francium oxides. This reaction would likely be exceptionally fast and potentially lead to spontaneous ignition.
-
Reaction with Halogens: Francium would react violently with halogens (like chlorine, bromine, and iodine) to form francium halides. These reactions would be characterized by the rapid transfer of electrons from francium to the halogens.
-
Formation of Compounds: Francium would readily form compounds with various other elements and molecules, readily participating in ionic bonding due to its propensity to lose its single valence electron and form a +1 ion.
Applications (or Lack Thereof) of Francium
Given its extreme rarity and short half-life, francium currently lacks any significant practical applications. Its radioactive nature and rapid decay prevent its use in any widespread industrial or technological processes. Research involving francium is primarily focused on understanding its fundamental properties and nuclear behavior, rather than on practical applications. However, its unique properties make it a valuable subject for theoretical studies in atomic physics and nuclear chemistry.
Conclusion: The Reigning Champion of Reactivity
Francium reigns supreme as the most reactive metal on the periodic table. Its exceptionally low ionization energy, large atomic radius, and significant shielding effect combine to make it incredibly prone to losing its single valence electron. While its short half-life and rarity limit practical applications, francium's extreme reactivity serves as a compelling demonstration of the periodic trends and the principles that govern chemical behavior. Further theoretical and experimental studies, though challenging, continue to deepen our understanding of this fascinating and elusive element. The quest to unravel its secrets continues to push the boundaries of chemical and nuclear research, solidifying its position as a captivating element at the forefront of scientific exploration.
Latest Posts
Latest Posts
-
1 3 To The Power Of 3
Mar 31, 2025
-
Least Common Multiple For 18 And 24
Mar 31, 2025
-
What Is 3 4 Equivalent To In Fractions
Mar 31, 2025
-
Where Is Most Freshwater Found On Earth
Mar 31, 2025
-
Simplify The Square Root Of 512
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Most Reactive Metal On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
