Melting Point Of Meso Stilbene Dibromide
listenit
Mar 29, 2025 · 6 min read
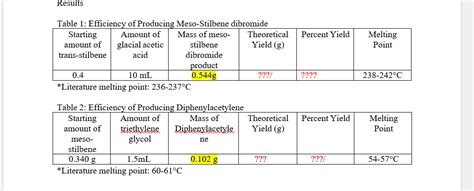
Table of Contents
Melting Point of Meso Stilbene Dibromide: A Comprehensive Analysis
The determination of melting point is a fundamental technique in organic chemistry, used for compound identification and purity assessment. Meso stilbene dibromide, a diastereomer of stilbene dibromide, presents an interesting case study due to its unique stereochemistry and resulting physical properties. This article delves deep into the melting point of meso stilbene dibromide, exploring the factors influencing it and the methods used for its accurate determination. We'll also examine its significance in the broader context of organic chemistry and its applications.
Understanding Meso Stilbene Dibromide
Before we delve into the melting point, let's establish a firm understanding of the compound itself. Stilbene dibromide is the product of the addition of bromine across the double bond of stilbene. Stilbene exists as two isomers: cis-stilbene and trans-stilbene. Bromination of either isomer leads to the formation of two diastereomers: a racemic mixture (dl-stilbene dibromide) and a meso compound (meso-stilbene dibromide). The key difference lies in the spatial arrangement of the bromine atoms and the phenyl groups.
Stereochemistry and its Impact
The meso isomer possesses an internal plane of symmetry, rendering it achiral despite having chiral centers. This internal symmetry significantly influences its physical properties, including its melting point. The racemic mixture, conversely, lacks this internal symmetry and exists as a mixture of two enantiomers, leading to different properties.
The crucial point here is that the melting point of meso-stilbene dibromide is distinctly different from that of the racemic mixture. This difference arises because of the intermolecular forces at play within the crystal lattice. The symmetrical nature of the meso isomer allows for more efficient packing in the solid state, influencing its melting point.
Determining the Melting Point: Experimental Techniques
Accurately measuring the melting point of meso stilbene dibromide requires precise experimental techniques. Several methods exist, each with its strengths and limitations.
Capillary Method
This is the most common and straightforward method. A small amount of the finely powdered sample is packed into a thin-walled glass capillary tube. The tube is then carefully placed in a melting point apparatus, typically a heated block or oil bath equipped with a thermometer. The temperature is gradually increased, and the observer notes the temperature range at which the solid begins to melt and the temperature at which the melting process is complete.
Challenges: The capillary method is susceptible to errors due to impurities in the sample, uneven heating, and the observer's subjective interpretation of the melting range. The rate of heating is crucial; too rapid heating can lead to inaccurate results.
Kofler Bench Method
The Kofler bench offers a more advanced approach. This method employs a heated metal plate with a temperature gradient across its surface. A small amount of the sample is placed on the hot plate, and its melting point is determined by observing the movement of the melting boundary across the sample.
Advantages: This method allows for the simultaneous observation of several samples, reducing the time required for analysis. The visual representation of the melting process can offer more insight into the sample's purity.
Factors Affecting the Melting Point of Meso Stilbene Dibromide
Several factors can influence the experimentally determined melting point of meso stilbene dibromide. Understanding these factors is vital for accurate measurements and interpretation of the results.
Purity of the Sample
Impurities significantly depress the melting point of a substance. Even small amounts of impurities can broaden the melting range and lower the observed melting point. Therefore, meticulous purification techniques such as recrystallization are critical before melting point determination.
Rate of Heating
As previously mentioned, the rate of heating affects the observed melting point. Too rapid heating prevents thermal equilibrium and leads to a falsely high melting point. A slow, controlled heating rate ensures accuracy.
Presence of Solvent
Residual solvent molecules trapped within the crystal lattice can affect the intermolecular interactions and consequently the melting point. Thorough drying of the sample is essential to eliminate this influence.
Atmospheric Pressure
While the effect is usually minor, changes in atmospheric pressure can slightly affect the melting point. For precise work, controlling the pressure is important.
Calibration of the Thermometer/Apparatus
A correctly calibrated thermometer or apparatus is paramount for accurate results. Any deviation in the thermometer calibration will directly affect the melting point measurement.
Sample Packing in Capillary Tube
The way the sample is packed into the capillary tube can influence heat transfer. A tightly packed sample ensures uniform heating and reduces the chance of inaccurate readings.
The Reported Melting Point and its Significance
While precise values may vary slightly depending on the experimental conditions and the purity of the sample, the generally accepted melting point of meso-stilbene dibromide is around 237-238 °C. This specific melting point serves as a crucial identifier for the compound. Its deviation from the racemic mixture's melting point emphasizes the significance of stereochemistry in influencing physical properties.
Applications and Significance in Organic Chemistry
The synthesis and analysis of meso-stilbene dibromide have broad applications within organic chemistry:
- Stereochemistry Education: It serves as an excellent example to illustrate concepts of stereochemistry, diastereomers, and meso compounds. The contrasting melting points of the meso and racemic diastereomers are invaluable for demonstrating the impact of molecular symmetry.
- Organic Synthesis Intermediate: Meso-stilbene dibromide can act as an intermediate in various organic synthesis reactions. Its specific reactivity and functional groups allow it to participate in reactions such as elimination, substitution, and Grignard reactions, opening pathways for further chemical modifications.
- Crystallography Studies: The unique crystal structure of meso-stilbene dibromide makes it a suitable candidate for X-ray crystallographic studies, aiding in the understanding of molecular packing and intermolecular interactions.
- Pharmaceutical Research: Though not directly used in pharmaceuticals, the understanding of its properties contributes to the broader knowledge of similar compounds potentially relevant in drug discovery and development. The synthesis and properties of analogous compounds are routinely studied for their biological activity.
Conclusion
The melting point of meso-stilbene dibromide, typically around 237-238 °C, is a crucial physical property characterizing this unique diastereomer. Accurate determination of its melting point requires precise experimental techniques and careful consideration of various influencing factors, emphasizing the importance of sample purity, heating rate, and instrument calibration. The value and the methods used highlight the critical role of melting point determination in organic chemistry, both in compound identification and in the broader understanding of stereochemistry and molecular properties. Its applications extend beyond simple identification, serving as an educational tool and a potential intermediate in further organic synthesis. Understanding this seemingly simple measurement offers valuable insight into the complex world of organic molecules.
Latest Posts
Latest Posts
-
What Is The Percentage Of 0 125
Mar 31, 2025
-
How Are Clastic Sedimentary Rocks Classified
Mar 31, 2025
-
Is Melting Ice A Chemical Reaction
Mar 31, 2025
-
40 Is 32 Percent Of What Number
Mar 31, 2025
-
What Is The Highest Point Of A Transverse Wave Called
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Melting Point Of Meso Stilbene Dibromide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
