Maximum Number Of Electrons In P Orbital
listenit
Mar 29, 2025 · 5 min read
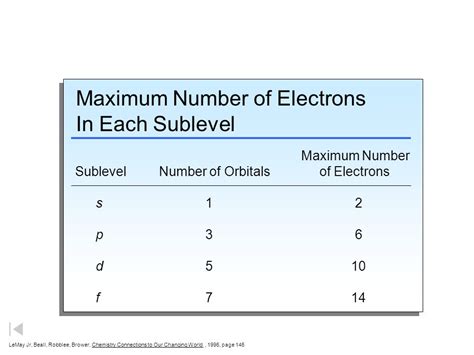
Table of Contents
Maximum Number of Electrons in a p Orbital: A Deep Dive
Understanding the maximum number of electrons a p orbital can hold is fundamental to grasping the basics of atomic structure and chemical bonding. This article provides a comprehensive exploration of this topic, delving into the quantum mechanical principles that govern electron distribution within atoms. We'll unpack the concepts of orbitals, electron shells, subshells, and the Pauli Exclusion Principle, all crucial to understanding the electron capacity of p orbitals. We will also discuss the implications of this electron capacity on chemical properties and reactivity.
Understanding Atomic Structure: Shells, Subshells, and Orbitals
Before we delve into the specifics of p orbitals, let's establish a solid foundation in atomic structure. Atoms consist of a nucleus containing protons and neutrons, surrounded by a cloud of electrons. These electrons are not randomly distributed but occupy specific regions of space called orbitals.
Electron Shells
Electrons are arranged in concentric shells or energy levels around the nucleus. These shells are designated by principal quantum numbers (n), with n = 1 representing the shell closest to the nucleus, n = 2 the next, and so on. Each shell can hold a maximum number of electrons, calculated using the formula 2n². Thus, the first shell (n=1) can hold a maximum of 2 electrons, the second shell (n=2) can hold 8 electrons, and so forth.
Electron Subshells
Within each electron shell, electrons are further organized into subshells. These subshells are identified by the azimuthal quantum number (l), which can have integer values ranging from 0 to n-1. Each value of l corresponds to a specific type of subshell:
- l = 0: s subshell (spherical in shape)
- l = 1: p subshell (dumbbell-shaped)
- l = 2: d subshell (more complex shapes)
- l = 3: f subshell (even more complex shapes)
Electron Orbitals
Each subshell is composed of one or more orbitals. Orbitals are regions of space where there's a high probability of finding an electron. The number of orbitals within a subshell is determined by the magnetic quantum number (ml), which can take integer values from -l to +l, including 0. Therefore:
- s subshell (l=0): Contains 1 orbital.
- p subshell (l=1): Contains 3 orbitals (ml = -1, 0, +1).
- d subshell (l=2): Contains 5 orbitals.
- f subshell (l=3): Contains 7 orbitals.
The p Subshell and its Orbitals: A Detailed Look
Now let's focus on the p subshell. As mentioned earlier, the p subshell has three orbitals, designated as px, py, and pz. These designations refer to their orientation in three-dimensional space: px along the x-axis, py along the y-axis, and pz along the z-axis. While these are visual representations to aid understanding, it's crucial to remember that these are probability distributions, not rigid boundaries.
The key takeaway: Each p orbital can hold a maximum of two electrons.
The Pauli Exclusion Principle: A Cornerstone of Atomic Structure
The Pauli Exclusion Principle is paramount to understanding electron configuration. It states that no two electrons within an atom can have the same set of four quantum numbers (n, l, ml, and ms). The spin quantum number (ms) can have only two values: +1/2 (spin up) and -1/2 (spin down).
This principle dictates that each orbital, regardless of its type (s, p, d, or f), can accommodate a maximum of two electrons, each with opposite spins. This is why a p orbital, being able to hold a maximum of two electrons, is essential in forming chemical bonds and shaping the properties of atoms.
Maximum Number of Electrons in a p Subshell
Since a p subshell contains three p orbitals (px, py, and pz), and each orbital can hold a maximum of two electrons, the maximum number of electrons a p subshell can hold is six. This is a crucial number in determining the electronic configuration of atoms and their chemical behavior. For instance, elements in the p-block of the periodic table have their outermost electrons in the p subshell, leading to a variety of bonding patterns and properties.
Implications of Electron Configuration on Chemical Properties
The electron configuration, including the number of electrons in the p subshell, significantly impacts an element's chemical properties. The outermost electrons, known as valence electrons, are directly involved in chemical bonding. Elements with partially filled p subshells tend to be highly reactive as they strive to achieve a stable electron configuration, often by gaining, losing, or sharing electrons to complete their outermost shell. This is the driving force behind many chemical reactions.
Examples: Electron Configuration and the p Subshell
Let's examine some examples to illustrate the role of the p subshell in determining electron configurations:
-
Nitrogen (N): Nitrogen has an atomic number of 7, meaning it has 7 electrons. Its electron configuration is 1s²2s²2p³. This shows three electrons in the 2p subshell, making nitrogen highly reactive.
-
Oxygen (O): Oxygen has an atomic number of 8, and its electron configuration is 1s²2s²2p⁴. It has four electrons in the 2p subshell, making it also quite reactive.
-
Neon (Ne): Neon has an atomic number of 10, and its electron configuration is 1s²2s²2p⁶. The 2p subshell is completely filled, making neon an inert gas—it is very unreactive.
These examples highlight how the number of electrons in the p subshell directly relates to an element's reactivity and overall chemical behavior.
Beyond the Basics: Advanced Concepts
While this article focuses on the fundamental aspects of p orbitals and electron capacity, understanding the nuances of quantum mechanics provides a richer picture. Concepts such as electron correlation, electron-electron repulsion, and the limitations of the simple orbital model add layers of complexity to the electron distribution within atoms and molecules.
Conclusion: The Significance of the p Orbital
Understanding the maximum number of electrons in a p orbital (two) and a p subshell (six) is essential for comprehending fundamental chemical concepts. This knowledge forms the basis for predicting chemical reactivity, bonding behavior, and the periodic properties of elements. The Pauli Exclusion Principle and the principles of quantum mechanics provide a framework for understanding the distribution and behavior of electrons within atoms, ultimately shaping the world around us. Further exploration of quantum chemistry will deepen your understanding of the intricate dance of electrons within atomic structures and their influence on chemical reactions. The seemingly simple idea of six electrons filling a p-subshell unlocks an understanding of a vast range of complex chemical phenomena.
Latest Posts
Latest Posts
-
What Is The Molar Mass Of Calcium Hydroxide
Mar 31, 2025
-
What Causes The Movement Of Lithospheric Plates
Mar 31, 2025
-
What Is The Driving Force Behind Plate Movement
Mar 31, 2025
-
What Is 3 11 As A Decimal
Mar 31, 2025
-
What Is The Square Root Of 841
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Maximum Number Of Electrons In P Orbital . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
