Mass Of An Electron In Amu
listenit
Mar 17, 2025 · 6 min read
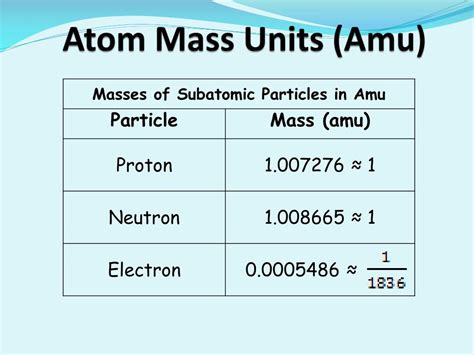
Table of Contents
The Mass of an Electron in AMU: A Deep Dive
The electron, a fundamental subatomic particle, carries a fundamental negative electric charge and is a key component in determining the properties of atoms and molecules. Understanding its mass, particularly expressed in atomic mass units (amu), is crucial for various scientific disciplines, from chemistry and physics to nuclear science and materials science. This article delves into the mass of an electron in amu, exploring its significance, measurement methods, and implications in different scientific contexts.
Understanding Atomic Mass Units (amu)
Before diving into the electron's mass, let's clarify the concept of atomic mass units (amu), also known as daltons (Da). An amu is defined as one-twelfth the mass of a single, unbound, neutral atom of carbon-12. This standardized unit provides a convenient scale for expressing the masses of atoms, isotopes, and subatomic particles. Using amu simplifies calculations and comparisons involving the masses of different particles, particularly in chemical reactions and nuclear processes.
Why amu instead of kilograms? While the kilogram is the standard unit of mass in the International System of Units (SI), using amu offers significant advantages when dealing with atomic and subatomic particles. The mass of these particles is exceptionally small when expressed in kilograms. Using amu normalizes these values to a more manageable scale, providing a clearer picture of their relative masses.
The Mass of an Electron in AMU: A Precise Measurement
The mass of an electron is approximately 9.109 × 10^-31 kilograms. However, expressing this in amu provides a more insightful perspective. Converting kilograms to amu requires using the conversion factor: 1 amu ≈ 1.6605 × 10^-27 kg.
Using this conversion, the mass of an electron in amu is calculated as:
(9.109 × 10^-31 kg) / (1.6605 × 10^-27 kg/amu) ≈ 0.00054858 amu
This is often rounded to 0.00055 amu for simplified calculations. It is important to remember that this is an approximate value, as the mass of the electron has been measured with extremely high precision, leading to numerous significant figures in more accurate representations. The slight variations seen in different sources are due to the precision of the measurements and the adopted values for fundamental constants.
Methods for Determining Electron Mass
The precise measurement of the electron's mass is a testament to the advancement of experimental physics. Various methods have been employed over the years, each contributing to increasingly accurate determinations. These techniques often involve cleverly manipulating the electron's behavior in electric and magnetic fields.
-
Millikan's Oil Drop Experiment: While not directly measuring the electron's mass, Robert Millikan's famous experiment (1909) determined the charge of an electron with impressive accuracy. Combining this charge measurement with other experimental data, such as the electron's charge-to-mass ratio (e/m), allowed scientists to calculate the electron's mass.
-
Spectroscopy: The study of electromagnetic spectra emitted or absorbed by atoms provides invaluable information about their structure and energy levels. Analyzing fine details in these spectra, including subtle shifts in energy levels due to the electron's mass, allows for precise mass calculations.
-
Particle Accelerators: Modern particle accelerators, such as cyclotrons and synchrotrons, propel charged particles to incredibly high speeds. By carefully analyzing the behavior of electrons at these speeds, and through techniques like precision mass spectrometry, we can determine their mass with even higher precision.
Significance of the Electron's Mass in AMU
The seemingly small mass of the electron in amu holds immense significance across various scientific disciplines:
1. Chemistry and Chemical Reactions
The electron's mass, while small, plays a significant role in chemical bonding and reactivity. The relative masses of electrons compared to protons and neutrons influence how atoms interact to form molecules. The mass difference contributes to kinetic energy differences and affects reaction rates and equilibrium positions. The isotopic masses of atoms, differing slightly due to differing neutron numbers, also have a subtle but measurable impact on reaction rates and equilibrium.
2. Nuclear Physics and Radioactivity
In nuclear processes, the electron's mass, while insignificant compared to nucleons (protons and neutrons), still impacts certain calculations. For instance, understanding the electron's mass is crucial when considering beta decay, where a neutron transforms into a proton, an electron (beta particle), and an antineutrino. The electron's mass is a part of the energy balance equation in this process.
3. Astrophysics and Cosmology
The electron's mass has profound implications for understanding the universe at large. Its mass contributes to the overall mass-energy density of the universe, influencing the expansion rate and the formation of large-scale structures. Moreover, the behavior of electrons in extreme environments, such as those found in stars and black holes, is vital for understanding astrophysical phenomena.
4. Materials Science and Nanotechnology
The electron's mass and its interaction with other particles influence the physical and chemical properties of materials. For instance, the behavior of electrons in solids determines their electrical conductivity and other material properties. At the nanoscale, the electron's mass becomes increasingly important in understanding quantum effects and designing advanced nanomaterials.
Relativistic Effects and Electron Mass
At very high speeds, approaching the speed of light, the electron's mass increases due to relativistic effects, as predicted by Einstein's theory of special relativity. This relativistic mass increase is not a change in the intrinsic mass of the electron, but rather a consequence of its increased energy. This increase in relativistic mass becomes significant only at velocities approaching the speed of light, and is a crucial consideration in high-energy physics experiments.
The Future of Electron Mass Measurement
While the electron's mass in amu is already known with remarkable precision, scientists continue to strive for even higher accuracy. Advancements in experimental techniques and theoretical understanding promise continued refinement of this fundamental constant. The search for ever greater precision is driven by the desire to better understand fundamental physical laws and to improve the accuracy of related calculations in various scientific fields.
Conclusion
The mass of an electron, seemingly minuscule when expressed in kilograms, takes on a new perspective when measured in atomic mass units (amu). This small mass plays a surprisingly significant role in various scientific disciplines, influencing chemical reactions, nuclear processes, astrophysical phenomena, and materials science. The continuing efforts to refine the measurement of this fundamental constant reflect its importance in our ongoing quest to understand the universe at its most basic level. From Millikan's groundbreaking experiment to modern particle accelerators, the journey to determine the electron's mass showcases the power of scientific inquiry and the precision achievable in modern physics. The ongoing refinement of its value underscores its enduring relevance in scientific endeavors across numerous disciplines.
Latest Posts
Latest Posts
-
How Do You Find The Secant Line
Mar 17, 2025
-
Is Water A Good Leaving Group
Mar 17, 2025
-
480 Cm Equals How Many M
Mar 17, 2025
-
What Is 8 In Fraction Form
Mar 17, 2025
-
The Elbow Is Proximal To The Shoulder
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Mass Of An Electron In Amu . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
