Location Of An Electron In An Atom
listenit
Mar 18, 2025 · 6 min read
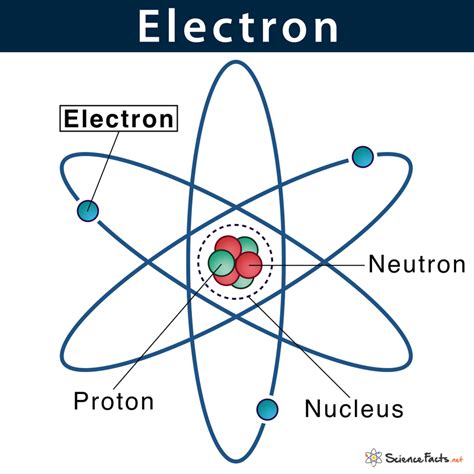
Table of Contents
The Elusive Location of an Electron in an Atom
The question of an electron's location within an atom has been a central theme in the development of modern physics. Unlike the relatively straightforward picture of planets orbiting a sun in our solar system, the behavior of electrons is governed by the bizarre and counterintuitive laws of quantum mechanics. There's no simple answer to "where is the electron?", and understanding the complexities requires delving into the core principles of atomic structure.
The Bohr Model: A Simplified (and Incorrect) Picture
Early attempts to model the atom, such as the Bohr model, presented a simplified, albeit ultimately inaccurate, picture. Bohr's model depicted electrons orbiting the nucleus in specific, defined energy levels or shells. This model suggested electrons occupied specific orbits, implying a relatively precise location. However, this model failed to account for many observed phenomena, most notably the spectra of more complex atoms.
Limitations of the Bohr Model
The Bohr model, while useful as a pedagogical tool, ultimately suffers from several critical flaws:
- Violation of Classical Physics: Electrons orbiting a nucleus, according to classical electromagnetism, should constantly emit radiation, losing energy and spiraling into the nucleus. Bohr's model arbitrarily postulates that this doesn't happen.
- Inaccuracy for Multi-Electron Atoms: The model works reasonably well for hydrogen (a single proton and a single electron), but its predictive power rapidly degrades as the number of electrons increases. The interactions between multiple electrons are not adequately addressed.
- Failure to Explain Fine Structure: The Bohr model couldn't explain the fine structure observed in atomic spectra – small but significant variations in spectral lines.
The Quantum Mechanical Approach: Probability Clouds and Orbitals
The shortcomings of the Bohr model led to the development of quantum mechanics, a radically different approach to understanding the atom. Instead of precise orbits, quantum mechanics describes electrons using wave functions. These wave functions don't directly tell us where an electron is, but rather the probability of finding it in a particular location.
The Uncertainty Principle: A Fundamental Limit
A cornerstone of quantum mechanics is Heisenberg's Uncertainty Principle. This principle states that it's fundamentally impossible to simultaneously know both the position and momentum of an electron (or any other particle) with perfect accuracy. The more precisely we know the electron's position, the less precisely we know its momentum, and vice-versa. This inherent uncertainty is not due to limitations in our measuring instruments but is a fundamental property of the universe at the quantum scale.
Atomic Orbitals: Regions of High Probability
Instead of orbits, quantum mechanics describes electrons occupying atomic orbitals. An atomic orbital is a region of space around the nucleus where there's a high probability of finding an electron. These orbitals are not sharply defined boundaries; instead, they represent probability distributions. The probability of finding an electron decreases as you move further away from the nucleus but never reaches exactly zero. This means there's a tiny, but non-zero, chance of finding an electron extremely far from the nucleus.
Shapes of Atomic Orbitals
Atomic orbitals have characteristic shapes, often depicted as three-dimensional probability density clouds. The simplest orbitals are the s orbitals, which are spherically symmetrical around the nucleus. The p orbitals are dumbbell-shaped, and more complex orbitals (d and f) exhibit even more intricate shapes. The shapes of these orbitals are determined by the electron's quantum numbers.
Quantum Numbers: Defining Electron States
Electrons in an atom are described by a set of four quantum numbers:
- Principal Quantum Number (n): This number determines the energy level of the electron and the size of the orbital. It can take positive integer values (1, 2, 3,...). Higher n values correspond to higher energy levels and larger orbitals.
- Azimuthal Quantum Number (l): This number specifies the shape of the orbital and can range from 0 to n-1. l=0 corresponds to s orbitals, l=1 to p orbitals, l=2 to d orbitals, and l=3 to f orbitals.
- Magnetic Quantum Number (ml): This number describes the orientation of the orbital in space. It can take integer values from -l to +l, including 0. For example, for p orbitals (l=1), ml can be -1, 0, or +1, representing three p orbitals oriented along the x, y, and z axes.
- Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, often referred to as its "spin." It can only take two values: +1/2 (spin up) or -1/2 (spin down).
These four quantum numbers uniquely define the state of an electron in an atom. No two electrons can have the same set of four quantum numbers, a principle known as the Pauli Exclusion Principle.
Electron Configuration and the Periodic Table
The arrangement of electrons in an atom, based on the principles of quantum mechanics and the Pauli Exclusion Principle, is called the electron configuration. This configuration determines the chemical properties of the element. The periodic table is organized based on electron configurations, with elements in the same column (group) having similar electron configurations in their outermost shell (valence electrons), thus exhibiting similar chemical behavior.
Valence Electrons and Chemical Bonding
The outermost electrons, the valence electrons, are particularly important in determining how atoms interact and form chemical bonds. They participate in the sharing or transfer of electrons, leading to the formation of molecules and compounds. The behavior of valence electrons, which reside in orbitals with the highest principal quantum number, directly impacts the reactivity and bonding properties of an element.
Beyond Atomic Orbitals: Molecular Orbitals
When atoms bond to form molecules, their atomic orbitals combine to create molecular orbitals. These molecular orbitals encompass the entire molecule and describe the probability distribution of electrons within the molecule. Understanding molecular orbitals is crucial for predicting the properties of molecules, including their stability, reactivity, and spectroscopic properties.
Delocalization of Electrons
In many molecules, electrons are not confined to individual atoms or specific bonds but are instead delocalized across the entire molecule. This delocalization plays a crucial role in determining the properties of conjugated systems, such as benzene, where electrons are shared equally among multiple carbon atoms. Delocalized electrons contribute to the enhanced stability and unique reactivity of these compounds.
Advanced Concepts and Techniques
The description of electron location continues to evolve with advancements in theoretical and experimental techniques. Sophisticated computational methods allow for increasingly accurate predictions of electron distribution in complex molecules and materials. Experimental techniques, like X-ray diffraction and photoelectron spectroscopy, provide invaluable data on electron density and orbital structure.
Conclusion: Probability, Not Precision
The location of an electron in an atom is not a simple matter of pinpointing its position. Instead, quantum mechanics provides a probabilistic description. We can talk about the probability of finding an electron in a particular region of space, described by atomic orbitals, but we cannot say with certainty where it is at any given moment. This probabilistic nature is a fundamental aspect of the quantum world and is crucial to understanding the behavior of matter at the atomic and molecular level. The more we delve into the quantum realm, the more we realize that precision gives way to probability, a paradigm shift that has revolutionized our understanding of the universe.
Latest Posts
Latest Posts
-
What Is 80 Percent Of 20
Mar 18, 2025
-
To Get Rid Of A Fraction Multiply By The
Mar 18, 2025
-
How To Factor X 3 2x 2 X 2
Mar 18, 2025
-
How Many 1 3 Cups Equals 1 Cup
Mar 18, 2025
-
What Is The Monomer Of A Lipid
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Location Of An Electron In An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
