Isotopes Of An Element Differ In Their
listenit
Mar 22, 2025 · 7 min read
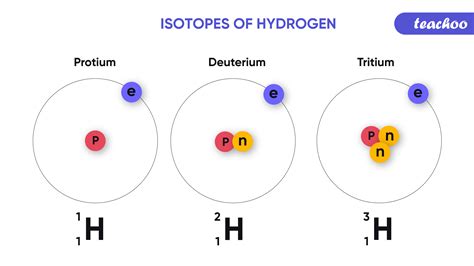
Table of Contents
Isotopes of an Element Differ in Their Neutron Number: A Deep Dive into Nuclear Structure and Isotopic Variations
Isotopes are variations of a chemical element that possess the same number of protons but differ in the number of neutrons within their atomic nuclei. This seemingly subtle difference profoundly impacts an element's properties, leading to a wide range of applications in various fields, from medicine and archaeology to nuclear energy and environmental science. Understanding the nuances of isotopic variation is crucial to grasping the complexities of nuclear physics and chemistry. This article delves deep into the topic, exploring the reasons behind isotopic differences, their implications, and their widespread use.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before exploring isotopic variations, it's vital to establish a solid understanding of basic atomic structure. An atom comprises three fundamental subatomic particles:
-
Protons: Positively charged particles residing within the atom's nucleus. The number of protons defines the element's atomic number and determines its identity on the periodic table. For example, all atoms with 6 protons are carbon atoms.
-
Neutrons: Neutrally charged particles also located in the nucleus. Unlike protons, the number of neutrons can vary within the same element, leading to the formation of isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons generally equals the number of protons in a neutral atom, ensuring electrical neutrality. However, atoms can gain or lose electrons to form ions, which carry a net electrical charge.
What Makes Isotopes Different? The Neutron Factor
The defining characteristic that differentiates isotopes of the same element is their neutron number. While the number of protons remains constant for a given element, the number of neutrons can vary. This variation results in different mass numbers, which represent the total number of protons and neutrons in the nucleus.
For example, consider carbon (atomic number 6). The most common isotope is carbon-12 (¹²C), containing 6 protons and 6 neutrons. However, carbon also exists as carbon-13 (¹³C) with 6 protons and 7 neutrons, and carbon-14 (¹⁴C) with 6 protons and 8 neutrons. All three are isotopes of carbon because they all possess 6 protons, but they differ in their neutron counts and, consequently, their mass numbers.
Notation and Representation of Isotopes
Isotopes are typically represented using the following notation:
- ¹²C: The superscript (12) indicates the mass number (protons + neutrons), and the subscript (6, often omitted) represents the atomic number (number of protons).
This notation clearly distinguishes different isotopes of the same element.
Isotopic Abundance and Natural Occurrence
Isotopes of an element rarely exist in equal proportions in nature. Instead, they exhibit varying abundances, with some isotopes being far more prevalent than others. This abundance is usually expressed as a percentage and is determined by factors such as nuclear stability and the processes that formed the element.
For example, ¹²C accounts for approximately 98.9% of naturally occurring carbon, while ¹³C makes up about 1.1%. ¹⁴C, a radioactive isotope used in radiocarbon dating, is present in trace amounts. The relative abundance of isotopes plays a critical role in determining the average atomic mass of an element as listed on the periodic table.
Impact of Neutron Number on Isotopic Properties
The difference in neutron number significantly influences several isotopic properties:
1. Nuclear Stability and Radioactivity
The ratio of protons to neutrons in an atom's nucleus plays a crucial role in its stability. Many isotopes are stable, meaning their nuclei remain intact indefinitely. However, some isotopes are radioactive, meaning their nuclei are unstable and undergo radioactive decay to achieve a more stable configuration. This decay involves the emission of particles or energy, transforming the nucleus into a different isotope or element. The neutron-to-proton ratio is a major determinant of radioactive decay modes, such as alpha decay, beta decay, and gamma decay. Isotopes with an excess of neutrons are more likely to undergo beta decay, while those with a deficiency of neutrons may undergo positron emission or electron capture.
2. Mass and Density
Isotopes with different neutron numbers have different masses. This mass difference, although seemingly small at the atomic level, can have measurable effects on macroscopic properties, such as density. Heavier isotopes generally lead to slightly higher densities in a substance.
3. Chemical Properties (Mostly Similar)
Despite the variations in nuclear structure, the chemical properties of isotopes of the same element are largely identical. This is because chemical reactions primarily involve the interaction of electrons, and the number of electrons is determined by the number of protons (atomic number), which remains constant for all isotopes of an element. However, subtle differences in reaction rates (kinetic isotope effects) can exist due to the mass difference between isotopes, particularly in reactions involving bond breaking or formation.
4. Physical Properties (Subtle Differences)
While chemical properties remain relatively constant, subtle variations can be observed in certain physical properties. For example, the vibrational frequencies of molecules containing different isotopes can differ, affecting spectroscopic properties. The boiling points and melting points can also experience minute variations due to the isotopic mass difference. These variations, though subtle, are measurable using sophisticated techniques.
Applications of Isotopes
The unique properties of isotopes find widespread applications across various scientific and technological domains:
1. Nuclear Medicine
Radioactive isotopes are extensively used in medical diagnostics and treatments. Techniques like Positron Emission Tomography (PET) scans utilize isotopes that emit positrons, providing detailed images of metabolic activity within the body. Radioactive isotopes are also employed in radiotherapy, targeting cancerous cells and destroying them using radiation. Examples include iodine-131 for thyroid treatment and cobalt-60 for external beam radiation therapy.
2. Radiocarbon Dating
Carbon-14 (¹⁴C), a radioactive isotope of carbon, is fundamental to radiocarbon dating, a technique used to determine the age of organic materials up to approximately 50,000 years old. The decay rate of ¹⁴C is constant, allowing scientists to estimate the time elapsed since the organism died based on the remaining ¹⁴C content. This technique is invaluable in archaeology, anthropology, and geology.
3. Environmental Science
Stable isotopes are valuable tools in environmental studies. For instance, the isotopic composition of water (analyzing the ratio of deuterium to hydrogen) can reveal information about water sources and movement patterns. Isotope analysis is also used to trace pollutants, study food webs, and understand climate change effects on ecosystems.
4. Nuclear Energy
Isotopes like uranium-235 and plutonium-239 are used as fuel in nuclear reactors, generating electricity through nuclear fission. The controlled chain reaction resulting from the fission of these isotopes releases enormous amounts of energy.
5. Industrial Applications
Isotopes find applications in various industrial processes. They are used in gauging the thickness of materials, tracing the flow of fluids in pipelines, and in other quality control measures.
6. Scientific Research
Isotopes are indispensable research tools across various scientific disciplines. They are used in studying chemical reaction mechanisms, investigating protein structure, and exploring biological processes at the molecular level. Isotopic tracers allow scientists to follow the fate of specific atoms or molecules within complex systems.
Conclusion
The differences between isotopes of an element stem from the variation in their neutron numbers, resulting in changes in their mass numbers and impacting their nuclear stability. While their chemical properties remain largely identical, subtle variations in physical properties and significant differences in nuclear stability can lead to a wide spectrum of applications. From medical diagnostics and treatments to dating ancient artifacts and understanding environmental processes, isotopes are crucial tools in numerous fields, highlighting the significant impact of this seemingly small nuclear difference. The ongoing research and development in isotopic technologies promise even more innovative applications in the years to come, reinforcing their importance in scientific advancement and technological progress. Understanding the intricacies of isotopes is fundamental to appreciating the complex world of nuclear science and its far-reaching consequences.
Latest Posts
Latest Posts
-
What Is 5 Percent Of 150
Mar 24, 2025
-
Similarities Between Ionic And Covalent Compounds
Mar 24, 2025
-
What Is The Least Common Multiple Of 18 And 24
Mar 24, 2025
-
Angles That Share A Vertex And A Common Side Are
Mar 24, 2025
-
Wood Rotting Physical Or Chemical Change
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Isotopes Of An Element Differ In Their . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
