Isotopes Of An Element Differ Due To The Number Of
listenit
Mar 14, 2025 · 6 min read
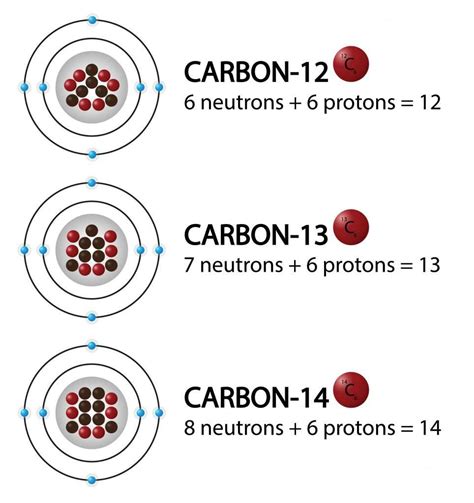
Table of Contents
Isotopes of an Element Differ Due to the Number of Neutrons
Isotopes are variations of a chemical element that share the same number of protons but differ in the number of neutrons. This difference in neutron count significantly impacts an element's properties, particularly its mass and stability. Understanding isotopes is crucial in various scientific fields, including nuclear physics, chemistry, and geology. This comprehensive article delves into the intricacies of isotopes, exploring their characteristics, applications, and significance in shaping our understanding of the universe.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before delving into the specifics of isotopes, let's refresh our understanding of atomic structure. An atom, the fundamental building block of matter, consists of three primary subatomic particles:
-
Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the atomic number of an element and determines its identity on the periodic table. For instance, all hydrogen atoms have one proton, all carbon atoms have six, and all oxygen atoms have eight.
-
Neutrons: Neutral particles (no charge) also residing in the atom's nucleus. Unlike protons, the number of neutrons in an atom can vary without changing the element's identity.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons typically equals the number of protons in a neutral atom, ensuring an overall neutral charge.
What are Isotopes? The Defining Difference
Isotopes are atoms of the same element that possess the same number of protons but differ in their number of neutrons. This difference in neutron count leads to variations in the atom's mass number (the sum of protons and neutrons). While isotopes share similar chemical properties due to their identical number of protons and electrons, their physical properties, particularly mass and nuclear stability, can differ significantly.
Mass Number and Atomic Mass
The mass number of an isotope represents the total number of protons and neutrons in its nucleus. It's denoted as a superscript to the left of the element's symbol (e.g., ¹²C for carbon-12). The atomic mass of an element, as listed on the periodic table, is a weighted average of the masses of all naturally occurring isotopes of that element. This weighted average accounts for the relative abundance of each isotope.
Isotopic Notation
Isotopes are typically represented using isotopic notation, which includes the mass number and the element's symbol. For example:
- ¹²C (Carbon-12): 6 protons, 6 neutrons
- ¹³C (Carbon-13): 6 protons, 7 neutrons
- ¹⁴C (Carbon-14): 6 protons, 8 neutrons
In all three cases, we are dealing with carbon atoms because they all have six protons. However, they differ in their number of neutrons, resulting in different mass numbers and properties.
Properties Influenced by Neutron Number
The varying number of neutrons significantly impacts several key properties of isotopes:
1. Mass
The most obvious difference between isotopes is their mass. Isotopes with more neutrons have a greater mass than those with fewer neutrons. This mass difference plays a vital role in various applications, such as mass spectrometry and radioisotope dating.
2. Nuclear Stability
The ratio of protons to neutrons in an atom's nucleus greatly influences its stability. Certain neutron-to-proton ratios lead to stable isotopes, while others result in unstable or radioactive isotopes. Radioactive isotopes undergo radioactive decay, emitting particles or energy to achieve a more stable configuration. This decay process is characterized by a specific half-life, the time it takes for half of the radioactive atoms in a sample to decay.
3. Radioactive Decay Modes
Unstable isotopes decay through various modes, including:
- Alpha decay: Emission of an alpha particle (two protons and two neutrons).
- Beta decay: Emission of a beta particle (an electron or a positron).
- Gamma decay: Emission of a gamma ray (high-energy photon).
The specific decay mode and the associated energy released depend on the specific isotope and its nuclear structure.
Applications of Isotopes
The unique properties of isotopes make them invaluable tools in various fields:
1. Medical Applications
Radioactive isotopes are widely used in medical diagnostics and treatment:
-
Medical Imaging: Technetium-99m is a commonly used radioactive tracer in nuclear medicine imaging techniques like SPECT (Single-Photon Emission Computed Tomography) and PET (Positron Emission Tomography) scans. These scans help visualize internal organs and detect abnormalities.
-
Radiation Therapy: Radioactive isotopes like cobalt-60 and iodine-131 are utilized in radiation therapy to target and destroy cancerous cells.
2. Industrial Applications
Isotopes find extensive use in various industrial applications:
-
Radioactive Tracers: Radioactive isotopes are used as tracers to track the movement of materials in industrial processes. This technique helps optimize processes and identify leaks or inefficiencies.
-
Gauging and Measurement: Isotope-based gauging techniques are employed to measure the thickness, density, or level of materials in various industrial settings.
3. Scientific Research
Isotopes are essential tools in scientific research, contributing to advancements in several areas:
-
Radiocarbon Dating: Carbon-14, a radioactive isotope of carbon, is used to date organic materials up to approximately 50,000 years old. This technique has revolutionized archaeology and paleontology.
-
Geological Dating: Other radioactive isotopes, such as uranium-238 and potassium-40, are used to date geological formations and estimate the age of the Earth.
-
Environmental Science: Isotopes are used to trace the movement of pollutants in the environment and study various environmental processes.
Isotope Separation Techniques
Separating isotopes is a complex process because isotopes of the same element have almost identical chemical properties. Several techniques are employed for isotope separation, including:
-
Gaseous Diffusion: This method exploits the slight differences in the diffusion rates of gaseous isotopes.
-
Centrifugation: Centrifuges are used to separate isotopes based on their mass differences. Heavier isotopes tend to migrate towards the outer edge of the centrifuge.
-
Laser Isotope Separation: This technique utilizes lasers to selectively excite and ionize specific isotopes, allowing for their separation.
Significance of Isotopes
The study of isotopes is crucial for several reasons:
-
Understanding Nuclear Physics: Isotopes provide insights into the fundamental nature of the atomic nucleus and the forces governing nuclear stability.
-
Advances in Technology: Isotope-based technologies have revolutionized various fields, impacting healthcare, industry, and scientific research.
-
Environmental Monitoring: Isotopes play a key role in environmental monitoring and pollution control.
-
Geological and Archaeological Dating: Isotopes have greatly enhanced our ability to date geological formations and archaeological artifacts.
-
Understanding the Origin and Evolution of the Universe: The isotopic composition of elements in meteorites and stars helps us understand the origin and evolution of the solar system and the universe.
Conclusion: A Multifaceted World of Isotopes
The seemingly small difference in neutron number between isotopes has profound implications for their properties and applications. From medical imaging to geological dating, isotopes play vital roles in numerous aspects of modern science and technology. Continued research into isotope behavior and separation techniques promises further advancements in various scientific and industrial fields, enhancing our understanding of the universe and improving our lives. The study of isotopes continues to be a vibrant and essential field of scientific inquiry, with ongoing discoveries that shape our understanding of matter and energy. The future undoubtedly holds even greater potential for the application of isotope science, contributing to numerous technological advancements and scientific breakthroughs.
Latest Posts
Latest Posts
-
What Is 30 Percent Of 80
Mar 14, 2025
-
How Many Ft In 100 Yards
Mar 14, 2025
-
How To Convert Kilograms Into Grams
Mar 14, 2025
-
Water Is Always A Product In What Type Of Reaction
Mar 14, 2025
-
What Is The Product Of Replication
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Isotopes Of An Element Differ Due To The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
