Is Oxygen More Electronegative Than Hydrogen
listenit
Mar 23, 2025 · 6 min read
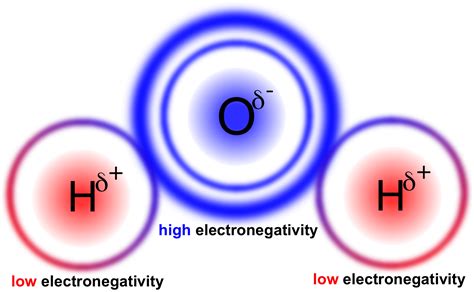
Table of Contents
Is Oxygen More Electronegative Than Hydrogen? A Deep Dive into Electronegativity
Electronegativity, a fundamental concept in chemistry, dictates how strongly an atom attracts electrons within a chemical bond. Understanding electronegativity differences is crucial for predicting bond polarity, molecular geometry, and the overall reactivity of molecules. A common comparison arises when considering oxygen and hydrogen, two elements ubiquitous in organic and inorganic chemistry. So, the question is: is oxygen more electronegative than hydrogen? The unequivocal answer is yes. But let's delve deeper into the reasons why, exploring the underlying principles and their implications.
Understanding Electronegativity
Before comparing oxygen and hydrogen, let's establish a firm grasp on the concept of electronegativity. It's a relative measure, meaning we don't assign absolute electronegativity values but rather compare elements based on their tendency to attract electrons. Several scales exist to quantify electronegativity, the most widely used being the Pauling scale. On this scale, fluorine, the most electronegative element, is assigned a value of 4.0. Other elements are then assigned values relative to fluorine.
Factors Influencing Electronegativity
Several factors influence an atom's electronegativity:
-
Nuclear Charge: A higher nuclear charge (more protons) attracts electrons more strongly. This means elements on the right side of the periodic table, with higher nuclear charges, tend to be more electronegative.
-
Atomic Radius: Smaller atoms have a greater electronegativity. This is because the valence electrons are closer to the nucleus and experience a stronger attractive force. The effective nuclear charge is higher for smaller atoms.
-
Shielding Effect: Inner electrons shield the outer (valence) electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge felt by valence electrons. Elements with fewer inner shells experience less shielding and exhibit greater electronegativity.
Comparing Oxygen and Hydrogen
Now, let's directly compare the electronegativities of oxygen and hydrogen.
Oxygen's Electronegativity
Oxygen (O) is located in Group 16 (or VIA) and Period 2 of the periodic table. Its atomic number is 8, meaning it has 8 protons. It has a relatively small atomic radius and a relatively high effective nuclear charge due to its compact electron configuration. These factors contribute to oxygen's strong attraction for electrons. On the Pauling scale, oxygen has an electronegativity of approximately 3.44.
Hydrogen's Electronegativity
Hydrogen (H) is located in Group 1 (or IA) and Period 1 of the periodic table. It has an atomic number of 1, with only one proton. Being the smallest atom, it has a relatively small atomic radius. However, it only has one electron, leading to a weaker attraction for additional electrons compared to oxygen. On the Pauling scale, hydrogen's electronegativity is approximately 2.20.
The Clear Difference
The difference in electronegativity between oxygen (3.44) and hydrogen (2.20) is significant. Oxygen's electronegativity is considerably higher, demonstrating its greater ability to attract electrons within a chemical bond.
Implications of the Electronegativity Difference
The significant electronegativity difference between oxygen and hydrogen has profound implications for the properties of molecules containing both elements, particularly water (H₂O).
Polar Covalent Bonds in Water
The O-H bonds in water are polar covalent bonds. This means that the shared electrons are not equally distributed between the oxygen and hydrogen atoms. The more electronegative oxygen atom pulls the shared electrons closer to itself, creating a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms. This charge separation creates a dipole moment, making water a polar molecule.
Consequences of Water's Polarity
Water's polarity is responsible for many of its unique properties, including:
-
High boiling point: The strong intermolecular forces (hydrogen bonds) between water molecules, arising from their polarity, require a significant amount of energy to overcome, resulting in a relatively high boiling point.
-
Excellent solvent: Water's polarity allows it to dissolve many ionic and polar compounds, making it an excellent solvent for biological processes and chemical reactions.
-
High surface tension: The strong hydrogen bonds between water molecules contribute to its high surface tension.
-
High specific heat capacity: Water's ability to absorb significant amounts of heat without a large temperature change is vital for temperature regulation in living organisms and in climate control.
Electronegativity in Other Oxygen-Hydrogen Compounds
The electronegativity difference between oxygen and hydrogen also influences the properties of other compounds containing both elements, such as alcohols, carboxylic acids, and carbohydrates. In these molecules, the O-H bonds are polar, contributing to the overall polarity and reactivity of the molecule.
Alcohols
Alcohols contain the hydroxyl group (-OH), where oxygen's higher electronegativity creates a polar bond. This polarity affects the solubility of alcohols in water and their ability to participate in hydrogen bonding.
Carboxylic Acids
Carboxylic acids contain the carboxyl group (-COOH), featuring both a carbonyl group (C=O) and a hydroxyl group (-OH). Both groups exhibit significant polarity due to the electronegativity difference between oxygen and carbon/hydrogen, leading to stronger intermolecular forces and higher boiling points compared to similar-sized molecules.
Carbohydrates
Carbohydrates are essential biomolecules composed of carbon, hydrogen, and oxygen. The presence of numerous O-H bonds contributes to their significant polarity and solubility in water. This water solubility is crucial for their roles in biological systems.
Beyond Oxygen and Hydrogen: Electronegativity Trends in the Periodic Table
Understanding the electronegativity difference between oxygen and hydrogen provides a foundation for understanding broader trends in the periodic table. Electronegativity generally increases across a period (from left to right) and decreases down a group (from top to bottom). This trend is directly linked to the factors discussed earlier – nuclear charge, atomic radius, and shielding.
Predicting Bond Polarity
The difference in electronegativity between two atoms in a bond can be used to predict the bond's polarity. A large electronegativity difference results in a highly polar bond, while a small difference indicates a less polar or nonpolar bond. This prediction is crucial in understanding molecular geometry and reactivity.
Applications in Various Fields
The concept of electronegativity and its impact on molecular properties finds applications in various fields:
-
Medicine: Understanding the polarity of drug molecules is crucial for their absorption, distribution, metabolism, and excretion in the body.
-
Materials Science: Electronegativity plays a significant role in the design of new materials with specific properties.
-
Environmental Science: The polarity of molecules affects their environmental behavior, such as their solubility in water and their potential for bioaccumulation.
Conclusion
In conclusion, oxygen is demonstrably more electronegative than hydrogen. This difference in electronegativity is a fundamental factor influencing the properties of numerous molecules, especially those containing O-H bonds. Understanding electronegativity is not just a theoretical exercise; it’s a practical tool for predicting molecular behavior and designing materials with desired properties across diverse scientific disciplines. The polarity resulting from this electronegativity difference is a cornerstone of chemistry, impacting everything from the unique properties of water to the design of life-saving drugs.
Latest Posts
Latest Posts
-
Calculate The Number Of Molecules In 4 00 Moles H2s
Mar 24, 2025
-
How Is An Ion Different From An Atom
Mar 24, 2025
-
What Percentage Is 12 Out Of 15
Mar 24, 2025
-
How Does The Cell Membrane Maintain Homeostasis
Mar 24, 2025
-
Is The Sun A Biotic Factor
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Is Oxygen More Electronegative Than Hydrogen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
