Is Iodine Chloride Ionic Or Covalent
listenit
Mar 15, 2025 · 5 min read
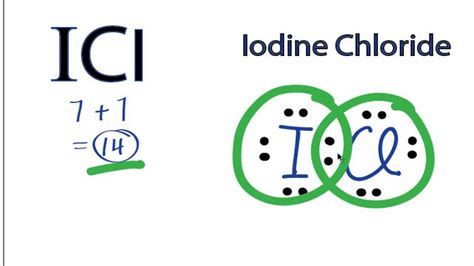
Table of Contents
Is Iodine Chloride Ionic or Covalent? A Deep Dive into Chemical Bonding
The question of whether iodine chloride (ICl) is ionic or covalent is a fascinating one, delving into the intricacies of chemical bonding and electronegativity. While a simplistic view might suggest a clear-cut answer, the reality is more nuanced. This article will explore the nature of bonding in ICl, examining its properties and comparing it to the characteristics of purely ionic and purely covalent compounds. We'll also discuss the factors influencing bond polarity and delve into the practical implications of understanding the bonding in ICl.
Understanding the Basics: Ionic vs. Covalent Bonds
Before diving into the specifics of ICl, let's refresh our understanding of ionic and covalent bonds.
Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. This typically occurs when a highly electronegative atom (like a halogen or oxygen) interacts with a highly electropositive atom (like an alkali or alkaline earth metal). The electronegativity difference is significant, resulting in one atom essentially donating an electron to the other, forming a cation (positive ion) and an anion (negative ion). These ions are then held together by strong coulombic forces. Classic examples include NaCl (sodium chloride) and MgO (magnesium oxide).
Covalent bonds, on the other hand, involve the sharing of electrons between atoms. This sharing often occurs between atoms with similar electronegativities, where neither atom has a strong enough pull to completely steal an electron from the other. The shared electrons form a bond that holds the atoms together. Purely covalent bonds are found in diatomic molecules like H₂ (hydrogen gas) or Cl₂ (chlorine gas), where the electrons are shared equally.
Electronegativity: The Key to Understanding Bond Type
Electronegativity is a crucial factor in determining the type of bond formed between two atoms. It measures an atom's ability to attract electrons towards itself in a chemical bond. The greater the electronegativity difference between two atoms, the more polar the bond will be. A large difference leads to ionic character, while a small difference indicates a covalent bond. However, it's important to remember that the line between ionic and covalent isn't always sharp; there's a spectrum of bond types, ranging from purely covalent to purely ionic, with many compounds falling somewhere in between – exhibiting polar covalent character.
Iodine Chloride (ICl): A Case Study
Iodine chloride (ICl) presents an interesting case study because it showcases the grey area between ionic and covalent bonding. Both iodine (I) and chlorine (Cl) are halogens, meaning they are highly electronegative. However, their electronegativities are not identical. Chlorine has a slightly higher electronegativity than iodine.
While the electronegativity difference isn't as dramatic as that seen in NaCl, it's not negligible either. This leads to a polar covalent bond in ICl. The chlorine atom attracts the shared electrons more strongly than the iodine atom, resulting in a partial negative charge (δ-) on the chlorine and a partial positive charge (δ+) on the iodine. This unequal sharing of electrons is what makes the bond polar.
Evidence for Polar Covalent Bonding in ICl:
-
Melting and Boiling Points: ICl has a relatively low melting point and boiling point compared to typical ionic compounds. Ionic compounds generally have much higher melting and boiling points due to the strong electrostatic forces between their ions. The lower melting and boiling points of ICl are consistent with a weaker, covalent type of bonding.
-
Solubility: ICl exhibits different solubility characteristics than purely ionic compounds. While ionic compounds are often soluble in polar solvents like water, the solubility of ICl is more complex and depends on the solvent. This nuanced behavior is typical of polar covalent compounds.
-
Conductivity: Unlike ionic compounds, which conduct electricity when molten or dissolved, ICl does not conduct electricity significantly in these states. This lack of conductivity points towards the absence of freely moving ions, a characteristic of covalent compounds.
-
Molecular Structure: ICl exists as discrete molecules, rather than an extended lattice of ions, further supporting a covalent bonding description. The molecules are not simply held together by ionic forces, but by the electron-sharing covalent bond.
The Spectrum of Bonding: Beyond Simple Categorization
It’s crucial to understand that the classification of a bond as purely ionic or purely covalent is a simplification. Most bonds lie somewhere along a continuum. The electronegativity difference provides a useful guideline, but other factors like the size of the atoms involved and the presence of multiple bonds can also influence the nature of the bond.
In ICl, while the bond is predominantly covalent, the electronegativity difference leads to a significant polar character. This means that the electron density is not evenly distributed, with a greater electron density around the more electronegative chlorine atom. This polarity affects the molecule's properties and reactivity.
Practical Implications and Further Considerations
Understanding the nature of bonding in ICl has important practical implications:
-
Reactivity: The polar nature of the ICl bond influences its reactivity. It can act as both an oxidizing agent (accepting electrons) and a reducing agent (donating electrons), depending on the reaction conditions. This versatility makes it useful in various chemical reactions and syntheses.
-
Applications: ICl finds applications in various fields, including organic chemistry as a reagent in halogenation reactions, and in the synthesis of certain iodine-containing compounds. Its properties, stemming from its polar covalent bonding, are crucial to these applications.
Conclusion: ICl – A Polar Covalent Compound
In conclusion, while the simple question of whether iodine chloride is ionic or covalent might initially seem straightforward, the answer is more nuanced. ICl exhibits a polar covalent bond, characterized by an unequal sharing of electrons due to the slight electronegativity difference between iodine and chlorine. This polarity significantly influences its properties and reactivity, distinguishing it from both purely ionic and purely covalent compounds. Understanding the spectrum of bonding and the interplay of factors like electronegativity is essential for predicting and explaining the behaviour of molecules like ICl. The slightly polar covalent nature of ICl underscores the complexity and beauty of chemical bonding, illustrating how the seemingly simple question of "ionic or covalent" often leads us to a deeper appreciation of the richness and subtlety of chemical interactions.
Latest Posts
Latest Posts
-
8x 4 4x 3 4 6x 4 4
Mar 15, 2025
-
What Do The Arrows On A Food Chain Represent
Mar 15, 2025
-
Is Salt An Element Compound Or Mixture
Mar 15, 2025
-
How Many Millimeters Are In One Meter
Mar 15, 2025
-
What Is 1 2 3 8
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is Iodine Chloride Ionic Or Covalent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
