Is Freezing Water Endothermic Or Exothermic
listenit
Mar 27, 2025 · 5 min read
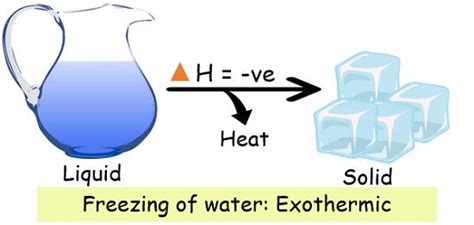
Table of Contents
Is Freezing Water Endothermic or Exothermic? Understanding Enthalpy Changes in Phase Transitions
The question of whether freezing water is endothermic or exothermic often trips up students and even seasoned science enthusiasts. The confusion stems from a misunderstanding of the subtle difference between heat flow and enthalpy change. While it might seem counterintuitive, the freezing of water is actually an exothermic process. Let's delve into the details to fully understand why.
Understanding Endothermic and Exothermic Processes
Before we tackle the specifics of water freezing, let's establish a solid foundation in the concepts of endothermic and exothermic reactions. These terms describe the direction of heat flow during a process:
-
Exothermic Processes: These processes release heat into their surroundings. The system's enthalpy (total heat content) decreases, and the surroundings become warmer. Think of combustion – burning wood releases heat, warming the air around it. Other examples include neutralization reactions and many forms of decomposition.
-
Endothermic Processes: These processes absorb heat from their surroundings. The system's enthalpy increases, and the surroundings become cooler. A classic example is dissolving ammonium nitrate in water – the solution becomes noticeably colder. Photosynthesis is another excellent example of an endothermic process.
The Enthalpy of Fusion and Freezing: A Closer Look
The key to understanding the freezing of water lies in the concept of enthalpy of fusion, also known as the latent heat of fusion. This represents the amount of heat energy required to change one mole of a substance from a solid to a liquid at its melting point. The opposite process, freezing (liquid to solid), involves the release of the same amount of heat energy.
Because energy must be removed from the water molecules for them to slow down enough to form the rigid structure of ice, the freezing process is exothermic. The energy released during freezing is exactly equal to the energy absorbed during melting (at the same temperature and pressure).
Molecular Perspective on Freezing
Imagine water molecules in their liquid state. They are moving around relatively freely, colliding with each other constantly. As the temperature drops, these molecules lose kinetic energy and move slower. Below 0°C (32°F), the attractive forces between the water molecules become dominant. They begin to arrange themselves into a regular, crystalline structure – ice.
This formation of a structured, ordered lattice requires the water molecules to release energy. This released energy is the enthalpy of fusion, making the process exothermic. The energy is not simply disappearing; it's transferred to the surrounding environment, which is why you might feel a slightly warmer area near a newly formed ice cube.
Why the Confusion? The Role of Heat Transfer
The confusion surrounding the exothermic nature of freezing often arises because we're observing a drop in temperature. We naturally associate temperature decrease with heat loss. While it's true that the water is losing heat, it's crucial to remember that the heat is being released into the surroundings, not simply disappearing. This released heat is the hallmark of an exothermic process.
Consider this: To freeze water, you need to remove heat from it. This removal of heat is what causes the temperature to decrease. However, the act of the water molecules reorganizing themselves into ice releases heat into the surroundings. The temperature change of the water is a consequence of heat transfer; the exothermic nature is determined by the direction of heat flow in the system.
Practical Applications and Real-World Examples
The exothermic nature of freezing has numerous practical applications:
-
Ice Packs: Instant cold packs often rely on the endothermic process of dissolving ammonium nitrate, but the ice packs we use for injuries rely on the exothermic release of heat as water freezes inside a gel pack.
-
Freezing Food: Freezing food relies on the exothermic release of heat to preserve food. The removal of heat lowers the temperature, slowing down microbial growth and enzymatic activity.
-
Weather Phenomena: The freezing of water in the atmosphere contributes to weather phenomena like the formation of frost and ice crystals in clouds. The release of heat during this process plays a role in atmospheric dynamics.
-
Industrial Processes: Many industrial processes utilize controlled freezing for applications such as cryopreservation (freezing biological materials) and the production of certain materials with specific crystalline structures.
Differentiating Between Heat Transfer and Enthalpy Change
It’s essential to maintain a clear distinction between heat transfer and enthalpy change. Heat transfer refers to the movement of heat energy between a system and its surroundings. Enthalpy change focuses on the change in the total heat content of a system during a process. Freezing is exothermic because the enthalpy of the system (water) decreases as heat is released to the surroundings, even though heat is being transferred out of the water.
Addressing Common Misconceptions
Many misconceptions arise when discussing the freezing of water. Here are some crucial clarifications:
-
Temperature vs. Heat: Temperature is a measure of the average kinetic energy of molecules. Heat is the transfer of energy due to a temperature difference. While freezing causes a temperature decrease in the water, the process itself is exothermic due to the release of heat.
-
Phase Transition: Freezing is a phase transition, a change of state from liquid to solid. Phase transitions always involve heat transfer, and the direction of heat transfer dictates whether the process is endothermic or exothermic.
-
Entropy: The freezing process is accompanied by a decrease in entropy (disorder). Exothermic processes generally lead to a decrease in entropy of the system, while endothermic processes usually increase entropy.
Conclusion: Freezing – An Exothermic Process
Freezing water is an exothermic process. While the temperature of the water decreases, the process itself releases heat into the surroundings, signifying an exothermic change in enthalpy. Understanding this distinction between heat transfer and enthalpy change is crucial for grasping the fundamental principles of thermodynamics and their diverse applications in various scientific and technological fields. The key is to focus on the direction of heat flow within the system, not the overall temperature change. This seemingly simple process illustrates the complex interplay of energy and matter at a molecular level, highlighting the importance of precise terminology and a thorough understanding of thermodynamic principles.
Latest Posts
Latest Posts
-
How Many Sides Are There In A Dodecagon
Mar 30, 2025
-
Divides The Body Into Anterior And Posterior Sections
Mar 30, 2025
-
Lim As X Approaches Negative Infinity
Mar 30, 2025
-
How To Factor X 3 2x 2
Mar 30, 2025
-
What Is The Value Of 32 4
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Is Freezing Water Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
