In The Periodic Table Horizontal Rows Are Called
listenit
Mar 28, 2025 · 7 min read
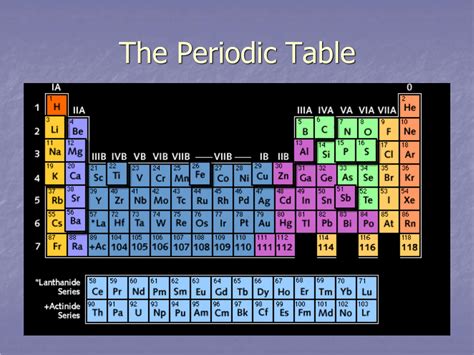
Table of Contents
In the Periodic Table, Horizontal Rows are Called Periods: A Deep Dive into the Organization of Elements
The periodic table, a cornerstone of chemistry, organizes the chemical elements in a structured manner that reflects their atomic structure and properties. Understanding its organization is crucial for grasping fundamental chemical concepts. One key aspect of this organization lies in the arrangement of elements into horizontal rows, known as periods. This article delves into the significance of periods in the periodic table, exploring their relationship to electron shells, recurring properties, and the overall structure of this indispensable scientific tool.
What are Periods in the Periodic Table?
The horizontal rows in the periodic table are called periods. Each period represents a principal energy level or shell in which electrons orbit the atom's nucleus. As we move from left to right across a period, the atomic number increases, meaning that each successive element has one more proton and, consequently, one more electron. These additional electrons fill the available orbitals within that specific energy level.
The Significance of Electron Shells
The number of periods corresponds directly to the number of electron shells filled in the atoms of elements within that period. For instance:
- Period 1: Contains only hydrogen (H) and helium (He), which have electrons filling the first energy level (n=1), which can only hold a maximum of two electrons.
- Period 2: Includes elements from lithium (Li) to neon (Ne). Their electrons fill the second energy level (n=2), which can accommodate up to eight electrons.
- Period 3: Extends from sodium (Na) to argon (Ar), and their electrons fill the third energy level (n=3), also with a maximum capacity of eight electrons.
This pattern continues, with each subsequent period representing a higher energy level and increasing electron capacity, although the specifics of electron shell filling become more complex with the introduction of d and f orbitals in higher periods.
Periodicity of Properties: A Key Feature of Periods
The arrangement of elements in periods reveals a fascinating phenomenon: periodicity. As we traverse a period, the chemical and physical properties of elements exhibit a cyclical pattern. This trend is closely linked to the progressive filling of electron shells.
Trends in Atomic Radius
Atomic radius, the distance from the nucleus to the outermost electron, generally decreases across a period. This is because the increasing nuclear charge (more protons) attracts the electrons more strongly, pulling them closer to the nucleus. Although more electrons are added, they are all within the same energy level and the added shielding effect is less significant than the increased nuclear pull.
Trends in Ionization Energy
Ionization energy is the energy required to remove an electron from a neutral atom. Ionization energy generally increases across a period. The increased nuclear charge holds the electrons more tightly, making it more difficult to remove them.
Trends in Electronegativity
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Electronegativity generally increases across a period for similar reasons as ionization energy: the stronger nuclear pull makes the atom more capable of attracting electrons from other atoms.
Trends in Metallic Character
Metallic character, which refers to properties such as electrical conductivity, malleability, and ductility, generally decreases across a period. Elements on the left side of a period are typically metals, while elements on the right tend to be non-metals. This trend reflects the increasing tendency for atoms to gain electrons (forming negative ions) rather than lose electrons (forming positive ions) as we move from left to right.
The Structure and Length of Periods: A Closer Look
The length of each period is determined by the number of electrons that can occupy the electron shells being filled in that period. The first two periods are short, containing only 2 and 8 elements, respectively. This is because the first energy level (n=1) can hold only two electrons (in the 1s orbital) and the second energy level (n=2) can hold a maximum of eight electrons (in the 2s and 2p orbitals).
Periods 3 and 4 each contain 8 elements, reflecting the same maximum capacity of the third (n=3) and fourth (n=4) energy levels. However, from Period 4 onwards, the pattern becomes more complex. This complexity arises due to the filling of d and f orbitals, which are lower in energy than the next principal energy level. This results in the longer periods which are seen as we progress down the periodic table.
The Longer Periods and Subshells
Periods 4-7 are much longer than the first three. This length stems from the filling of the d and f orbitals.
- Periods 4 and 5: These periods have 18 elements due to the filling of the 3d orbitals (which have 10 electrons) before the 4p and 5p orbitals are filled.
- Periods 6 and 7: These are the longest periods, containing 32 elements each. This is because the 4f orbitals (with 14 electrons) are filled before the 5d orbitals and 6d orbitals are filled, resulting in the lanthanides and actinides series which are usually placed separately at the bottom of the periodic table for clarity.
The Importance of Periods in Predicting Chemical Behavior
The periodic arrangement based on periods allows us to predict and understand the chemical behavior of elements. The periodicity of properties implies that elements within the same group or column often exhibit similar properties, due to the similar electron configuration in their outermost shells (valence electrons). Likewise, the trend of properties across a period helps explain the reactivity differences observed between elements.
Example: Reactivity across Period 3
Consider Period 3, ranging from sodium (Na) to argon (Ar). Sodium, at the far left, is highly reactive because it readily loses one electron to achieve a stable electron configuration. Chlorine (Cl), toward the right, is also highly reactive but in the opposite way; it readily gains one electron to achieve a stable configuration. Argon, a noble gas at the far right, is virtually inert because its outer electron shell is already full. This trend in reactivity clearly illustrates the influence of electron configuration and the predictable changes that occur across a period.
Periods and the Development of the Periodic Table
The concept of periods was central to the development of the modern periodic table. Early attempts at organizing elements were based on observing recurring patterns in properties. Dmitri Mendeleev's contribution was instrumental, as he arranged elements based on increasing atomic weight and observed the periodic recurrence of properties. This allowed him to predict the existence and properties of elements that were yet to be discovered, significantly enhancing the predictive power of the periodic table. The subsequent understanding of atomic structure and electron configuration solidified the importance of periods in the table's organization.
Periods and Beyond: Further Exploration
The significance of periods extends beyond simply organizing elements. It provides a framework for:
- Understanding Chemical Bonding: The location of an element within a period helps determine its bonding behavior, such as the type of bonds it forms (ionic, covalent, metallic).
- Predicting Chemical Reactions: The periodic trends within a period allow chemists to predict the outcome of many chemical reactions with reasonable accuracy.
- Material Science: The properties of elements and their periodic relationships are fundamental to material science and engineering, allowing for the design and development of new materials with specific properties.
Conclusion: Periods as the Foundation of Chemical Understanding
In conclusion, the horizontal rows—the periods—within the periodic table are far more than just a convenient arrangement of elements. They represent the fundamental energy levels of atoms, dictate periodic trends in properties, and provide a powerful framework for understanding and predicting chemical behavior. The concept of periods is fundamental to the structure and function of the periodic table, highlighting its pivotal role in chemistry and related scientific fields. Appreciating the significance of periods offers a profound insight into the intricate organization and underlying principles governing the behavior of matter at an atomic level. From predicting reactivity to understanding bonding, periods provide the crucial foundation for advanced chemical concepts and applications.
Latest Posts
Latest Posts
-
What Number Is 10 Of 20
Mar 31, 2025
-
X 3 2x 2 X 4
Mar 31, 2025
-
How Is Magma Created In A Subduction Zone
Mar 31, 2025
-
What Is The Least Reactive Element
Mar 31, 2025
-
How To Subtract Negative And Positive Fractions
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about In The Periodic Table Horizontal Rows Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
