If Gibbs Free Energy Is Negative
listenit
Apr 06, 2025 · 6 min read
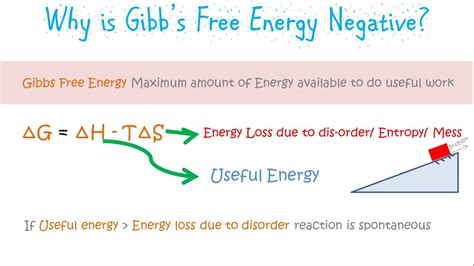
Table of Contents
If Gibbs Free Energy is Negative: A Deep Dive into Spontaneity and Thermodynamics
Gibbs Free Energy (ΔG), a thermodynamic potential, is a crucial concept in chemistry and physics, providing insights into the spontaneity of a process at constant temperature and pressure. Understanding its implications, particularly when it's negative, is vital for comprehending various phenomena, from chemical reactions to phase transitions. This comprehensive article delves into the meaning of a negative Gibbs Free Energy, its implications for spontaneity, equilibrium, and the factors influencing its value.
Understanding Gibbs Free Energy
Before exploring the significance of a negative ΔG, let's establish a firm understanding of Gibbs Free Energy itself. Defined as:
ΔG = ΔH - TΔS
Where:
- ΔG represents the change in Gibbs Free Energy
- ΔH represents the change in enthalpy (heat content) of the system
- T represents the absolute temperature in Kelvin
- ΔS represents the change in entropy (disorder) of the system
This equation beautifully encapsulates the interplay between enthalpy and entropy, two fundamental thermodynamic properties. Enthalpy reflects the system's heat content; a negative ΔH signifies an exothermic process (releasing heat), while a positive ΔH indicates an endothermic process (absorbing heat). Entropy, on the other hand, reflects the degree of disorder or randomness within the system; a positive ΔS signifies an increase in disorder, while a negative ΔS represents a decrease in disorder.
The Significance of a Negative Gibbs Free Energy
The core significance of Gibbs Free Energy lies in its predictive power regarding the spontaneity of a process. A negative ΔG indicates that a process will occur spontaneously under constant temperature and pressure conditions. This doesn't necessarily mean the process will be fast; spontaneity refers to the thermodynamic likelihood of the process occurring, not its kinetics. A negative ΔG simply implies that the process is energetically favorable and will proceed without external intervention.
Spontaneity and Equilibrium
The relationship between ΔG and spontaneity is pivotal:
- ΔG < 0 (Negative): The process is spontaneous; it will proceed in the forward direction without external input.
- ΔG > 0 (Positive): The process is non-spontaneous; it will not proceed in the forward direction without external input. The reverse process will be spontaneous.
- ΔG = 0 (Zero): The process is at equilibrium; the forward and reverse rates are equal, and there is no net change in the system's composition.
This understanding allows us to predict the direction of a reaction or the likelihood of a phase transition based solely on the Gibbs Free Energy change.
Factors Influencing Gibbs Free Energy and its Sign
Several factors influence the magnitude and sign of ΔG, making it a dynamic and context-dependent parameter:
1. Enthalpy (ΔH):
- Exothermic Reactions (ΔH < 0): These reactions release heat, contributing to a more negative ΔG, favoring spontaneity. The system's energy decreases, making the process more likely to occur.
- Endothermic Reactions (ΔH > 0): These reactions absorb heat, making a negative ΔG less likely. The system's energy increases, requiring external energy input to overcome this energy barrier.
2. Entropy (ΔS):
- Increase in Disorder (ΔS > 0): An increase in entropy generally favors spontaneity. Nature tends towards higher disorder; a more disordered state is statistically more probable. This positive contribution to ΔG makes a negative overall ΔG more likely, especially at higher temperatures.
- Decrease in Disorder (ΔS < 0): A decrease in entropy hinders spontaneity. Highly ordered states are less probable, requiring energy input to maintain order. This negative contribution to ΔG makes a negative overall ΔG less likely.
3. Temperature (T):
Temperature plays a crucial role, particularly in the context of entropy's contribution. The TΔS term becomes increasingly significant at higher temperatures.
- High Temperatures: At high temperatures, the TΔS term can dominate, even if ΔH is positive. If the entropy increase is substantial, a positive ΔH can be overcome, leading to a negative ΔG and spontaneity. This is often seen in phase transitions, where increased disorder at high temperatures favors melting or boiling.
- Low Temperatures: At low temperatures, the TΔS term is less significant. Spontaneity is primarily governed by ΔH. Exothermic reactions (ΔH < 0) are more likely to be spontaneous at low temperatures.
Real-World Applications of Negative Gibbs Free Energy
The concept of negative Gibbs Free Energy is not merely theoretical; it finds numerous practical applications across various scientific disciplines:
1. Chemical Reactions:
Predicting the spontaneity of chemical reactions is paramount in many areas, including:
- Industrial Chemistry: Designing efficient and spontaneous chemical processes for manufacturing products requires understanding ΔG to optimize reaction conditions.
- Environmental Chemistry: Assessing the spontaneity of environmental reactions helps predict pollution spread and remediation strategies.
- Biochemistry: Understanding metabolic pathways relies heavily on the spontaneity of biochemical reactions, governed by their ΔG values.
2. Phase Transitions:
Phase transitions, like melting, boiling, and sublimation, are intrinsically linked to Gibbs Free Energy. A negative ΔG indicates the thermodynamic feasibility of a particular phase transition at a given temperature and pressure. For example:
- Melting: Ice melting into water at temperatures above 0°C is spontaneous because ΔG is negative under these conditions.
- Boiling: Water boiling into steam at temperatures above 100°C is spontaneous due to a negative ΔG.
3. Material Science:
The stability and reactivity of materials are directly related to their Gibbs Free Energy. Predicting the spontaneity of material degradation or transformation is crucial for designing durable and reliable materials.
4. Electrochemistry:
Gibbs Free Energy is directly related to the cell potential (E) in electrochemical cells through the equation:
ΔG = -nFE
Where:
- n is the number of moles of electrons transferred
- F is Faraday's constant
A negative ΔG corresponds to a positive cell potential, indicating a spontaneous electrochemical reaction (like a battery discharging).
Beyond Spontaneity: Kinetics and Reaction Rate
It's crucial to emphasize that a negative ΔG only indicates the thermodynamic likelihood of a reaction; it says nothing about the kinetic feasibility or the reaction rate. A reaction with a highly negative ΔG might still be incredibly slow if it has a high activation energy barrier. Catalysts are often used to lower this activation energy, thereby increasing the reaction rate without altering the thermodynamic spontaneity (ΔG).
Conclusion: The Power of Negative Gibbs Free Energy
A negative Gibbs Free Energy signifies a thermodynamically spontaneous process at constant temperature and pressure. This fundamental concept provides a powerful tool for predicting the direction and likelihood of various phenomena, from chemical reactions to phase transitions, and finds wide-ranging applications in various scientific and engineering fields. Understanding the interplay between enthalpy, entropy, and temperature in determining the sign of ΔG is essential for comprehending and manipulating the behavior of systems. While a negative ΔG guarantees spontaneity, it's crucial to remember that the reaction rate is a separate consideration, often requiring catalytic intervention to accelerate inherently slow processes. The implications of a negative ΔG are profound, extending far beyond simple predictions to the design and optimization of countless real-world applications.
Latest Posts
Latest Posts
-
What Is The Atomic Number Of An Atom Equal To
Apr 07, 2025
-
At What Speed Does Every Type Of Electromagnetic Radiation Travel
Apr 07, 2025
-
6 To The Power Of 7
Apr 07, 2025
-
Greatest Common Factor 12 And 18
Apr 07, 2025
-
Write A Linear Function X With The Given Values
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about If Gibbs Free Energy Is Negative . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
