How To Turn Liquid Into Gas
listenit
Mar 16, 2025 · 5 min read
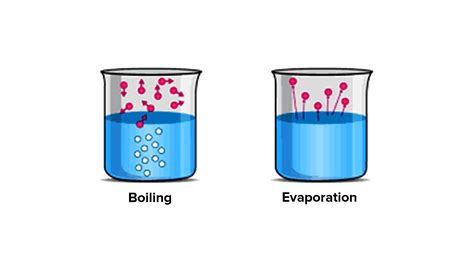
Table of Contents
How to Turn Liquid into Gas: A Comprehensive Guide to Vaporization
Turning a liquid into a gas, a process known as vaporization, is a fundamental concept in physics and chemistry with widespread applications in various industries. Understanding the principles behind this phase transition is crucial for many scientific and engineering endeavors. This comprehensive guide will delve into the different methods of vaporization, the factors influencing the process, and its practical applications.
Understanding the Phase Transition: Liquid to Gas
At a fundamental level, the transition from liquid to gas involves overcoming the intermolecular forces that hold liquid molecules together. In a liquid, these forces are relatively strong, keeping the molecules close together. However, by supplying sufficient energy, we can overcome these forces and allow the molecules to escape into the gaseous phase, where they are much farther apart and move more freely.
The Role of Temperature and Pressure
Temperature and pressure play pivotal roles in vaporization. Temperature directly affects the kinetic energy of the molecules. Higher temperatures mean molecules possess more kinetic energy, making it easier for them to overcome intermolecular forces and escape into the gaseous phase. Pressure, on the other hand, influences the rate at which molecules can escape. Lower pressure reduces the resistance that molecules face as they transition from liquid to gas, facilitating the process.
Types of Vaporization
There are three main types of vaporization:
-
Evaporation: This is a slow, surface-level process that occurs at temperatures below the boiling point. Molecules with higher kinetic energy near the surface of the liquid escape into the gaseous phase. Evaporation is influenced by factors such as temperature, surface area, and humidity. A warm, windy day will promote faster evaporation compared to a cool, humid day.
-
Boiling: This is a rapid vaporization process that occurs throughout the liquid when the temperature reaches the boiling point. At the boiling point, the vapor pressure of the liquid equals the external pressure, allowing bubbles of vapor to form within the liquid and rise to the surface. The boiling point varies depending on the liquid and the external pressure. Higher external pressure leads to a higher boiling point.
-
Sublimation (Indirect Vaporization): While not strictly a liquid-to-gas transition, sublimation involves a solid transforming directly into a gas without passing through the liquid phase. This occurs with substances like dry ice (solid carbon dioxide) under specific conditions of temperature and pressure. While not the focus of this article, it's relevant to understanding phase transitions generally.
Methods for Turning Liquid into Gas
Several methods facilitate the transformation of a liquid into a gas, each employing different principles and applications:
1. Heating
The most common method is simply heating the liquid. By increasing the temperature, we increase the kinetic energy of the molecules, enabling them to overcome intermolecular forces and escape into the gas phase. This method is utilized in various applications, including distillation, evaporation of water, and many industrial processes. The efficiency of heating depends on the heat transfer rate and the specific heat capacity of the liquid.
2. Reducing Pressure
Lowering the pressure above a liquid lowers the boiling point, making it easier for molecules to escape. This is often used in vacuum distillation, where liquids with high boiling points are vaporized at lower temperatures to prevent decomposition. Vacuum pumps are essential equipment in this method. This method is particularly crucial for handling heat-sensitive materials.
3. Using a Vacuum
A vacuum creates a very low pressure environment. This significantly reduces the boiling point of liquids, allowing for vaporization at much lower temperatures. Vacuum distillation is often used to purify or separate heat-sensitive materials.
4. Spraying or Atomization
Breaking a liquid into small droplets significantly increases the surface area exposed to the atmosphere. This enhances evaporation, as more molecules have the opportunity to escape from the surface. This technique is employed in spray dryers, which convert liquid solutions into powdered solids through rapid evaporation.
5. Mechanical Agitation
Stirring or agitating a liquid enhances the rate of evaporation. The constant movement brings more molecules to the surface, increasing their chance of escaping into the gaseous phase. This method is often combined with heating or reduced pressure for more efficient vaporization.
Factors Affecting the Rate of Vaporization
Several factors influence the speed at which a liquid turns into a gas:
- Temperature: Higher temperatures accelerate vaporization.
- Surface Area: A larger surface area allows more molecules to escape simultaneously.
- Pressure: Lower pressure facilitates vaporization.
- Humidity: Higher humidity slows down evaporation as the air is already saturated with water vapor.
- Wind: Wind removes the vaporized molecules from the vicinity of the liquid surface, accelerating evaporation.
- Nature of the Liquid: Different liquids have different intermolecular forces and boiling points, influencing their vaporization rates. Volatile liquids vaporize much faster than non-volatile ones.
Applications of Liquid-to-Gas Conversion
The conversion of liquids into gases is fundamental to numerous applications across various fields:
- Distillation: Widely used in the chemical industry to separate mixtures of liquids based on their different boiling points.
- Refrigeration: Refrigerants change phase between liquid and gas, absorbing heat during vaporization and releasing heat during condensation.
- Power Generation: Steam turbines in power plants utilize the vaporization of water to generate electricity.
- Drying Processes: Many industrial drying processes rely on evaporation to remove water or other solvents from materials.
- Aerosol Sprays: Liquids are vaporized and dispersed as fine droplets in aerosol cans.
- Medical Applications: Inhalers deliver medication in vaporized form for efficient lung absorption.
- Food Processing: Evaporation is used to concentrate liquids like milk and fruit juices.
- Weather Phenomena: Evaporation and condensation are integral to the water cycle and the formation of clouds and precipitation.
Conclusion: Mastering the Art of Vaporization
Turning a liquid into a gas is a ubiquitous process with far-reaching implications in various scientific and technological domains. By understanding the underlying principles, the different methods of vaporization, and the influencing factors, we can effectively control and utilize this phase transition for a wide range of applications. From industrial processes to everyday phenomena, the ability to manipulate the liquid-to-gas transition remains a cornerstone of scientific and technological advancement. Further research into optimizing these processes continues to yield innovations and improve efficiency across industries. The principles outlined in this guide provide a solid foundation for exploring the intricacies of this fundamental process and its remarkable applications.
Latest Posts
Latest Posts
-
What Is The Conjugate Acid Of Hco3
Mar 16, 2025
-
A Ma Or An Ma Degree
Mar 16, 2025
-
What Is 3 Square Root Of 3
Mar 16, 2025
-
5 1 2 To Improper Fraction
Mar 16, 2025
-
Graph Of X 1 X 1
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about How To Turn Liquid Into Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
