How To Find The Number Of Molecules In A Compound
listenit
Mar 28, 2025 · 6 min read
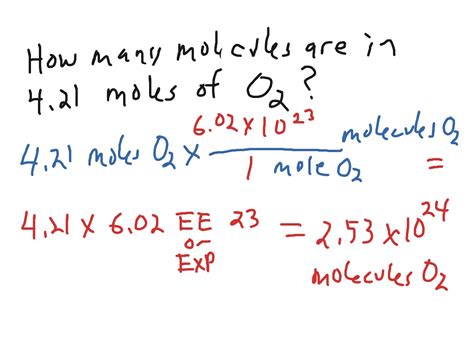
Table of Contents
How to Find the Number of Molecules in a Compound: A Comprehensive Guide
Determining the number of molecules in a given amount of compound is a fundamental concept in chemistry. This process, crucial for various chemical calculations and analyses, relies on understanding the relationship between moles, Avogadro's number, and the molar mass of the compound. This comprehensive guide will walk you through the steps, providing clear explanations and examples to solidify your understanding.
Understanding the Key Concepts
Before diving into the calculations, let's clarify the essential concepts:
1. The Mole (mol): A Chemist's Counting Unit
The mole is the cornerstone of chemical calculations. It's not a unit of mass or volume, but rather a unit representing a specific number of particles. One mole of any substance contains 6.022 x 10<sup>23</sup> particles. This enormous number is known as Avogadro's number (N<sub>A</sub>). Think of it as a chemist's equivalent of a dozen (12), but on a vastly larger scale. Instead of counting individual molecules, which is practically impossible, we work with moles.
2. Molar Mass (g/mol): Mass of One Mole
The molar mass of a compound is the mass of one mole of that substance, expressed in grams per mole (g/mol). It's calculated by summing the atomic masses (found on the periodic table) of all the atoms in the compound's chemical formula. For example, the molar mass of water (H₂O) is approximately 18 g/mol (2 x 1 g/mol for hydrogen + 1 x 16 g/mol for oxygen).
3. Avogadro's Number: The Bridge Between Moles and Molecules
Avogadro's number (N<sub>A</sub> = 6.022 x 10<sup>23</sup>) provides the crucial link between the macroscopic world (grams, moles) and the microscopic world (atoms, molecules). It tells us that one mole of any substance contains 6.022 x 10<sup>23</sup> molecules (or atoms, if it's a monatomic element).
Calculating the Number of Molecules
The process of finding the number of molecules involves a series of steps, often requiring conversions between mass, moles, and number of molecules. Let's break down the procedure:
Step 1: Determine the Molar Mass of the Compound
First, you need to know the chemical formula of the compound. Then, using the periodic table, find the atomic mass of each element present in the compound. Multiply each atomic mass by the number of atoms of that element in the formula and sum the results to obtain the molar mass.
Example: Let's find the molar mass of glucose (C₆H₁₂O₆).
- Carbon (C): Atomic mass ≈ 12.01 g/mol x 6 atoms = 72.06 g/mol
- Hydrogen (H): Atomic mass ≈ 1.01 g/mol x 12 atoms = 12.12 g/mol
- Oxygen (O): Atomic mass ≈ 16.00 g/mol x 6 atoms = 96.00 g/mol
Total Molar Mass of Glucose: 72.06 + 12.12 + 96.00 = 180.18 g/mol
Step 2: Convert Mass to Moles
If you're given the mass of the compound in grams, you need to convert it to moles using the molar mass. The formula for this conversion is:
Moles (mol) = Mass (g) / Molar Mass (g/mol)
Example: Let's say we have 10 grams of glucose.
Moles of glucose = 10 g / 180.18 g/mol ≈ 0.0555 mol
Step 3: Convert Moles to Number of Molecules
Finally, use Avogadro's number to convert the number of moles to the number of molecules. The formula is:
Number of Molecules = Moles (mol) x Avogadro's Number (6.022 x 10<sup>23</sup> molecules/mol)
Example: Continuing with our 10 grams of glucose (0.0555 mol):
Number of glucose molecules = 0.0555 mol x 6.022 x 10<sup>23</sup> molecules/mol ≈ 3.34 x 10<sup>22</sup> molecules
Working with Different Units and Scenarios
The process might vary slightly depending on the information provided. Let's explore some common scenarios:
Scenario 1: Given the Number of Moles
If the problem already provides the number of moles, you can skip Step 2 and directly proceed to Step 3 to calculate the number of molecules using Avogadro's number.
Scenario 2: Dealing with Gases Using the Ideal Gas Law
For gases, you can use the Ideal Gas Law (PV = nRT) to determine the number of moles (n) first, and then follow Steps 3. Here, P is pressure, V is volume, R is the ideal gas constant, and T is temperature. You'll need to ensure consistent units throughout the calculation.
Scenario 3: Working with Concentrations (Molarity)
If the concentration (molarity) of a solution is given, you can use it to find the number of moles in a specific volume of the solution. Molarity (M) is defined as moles of solute per liter of solution. You would first calculate the moles and then use Avogadro's number.
Example: A 2.0 M solution of sodium chloride (NaCl) has a volume of 500 mL.
Moles of NaCl = Molarity x Volume (in Liters) = 2.0 mol/L x 0.500 L = 1.0 mol
Number of NaCl molecules = 1.0 mol x 6.022 x 10<sup>23</sup> molecules/mol = 6.022 x 10<sup>23</sup> molecules
Advanced Considerations and Potential Challenges
While the basic calculation is straightforward, several factors can influence the accuracy and complexity:
-
Significant Figures: Pay close attention to significant figures throughout your calculations to avoid introducing unnecessary errors. The final answer should reflect the precision of the initial data.
-
Isotopes: The atomic masses used in calculating molar mass are usually weighted averages of the naturally occurring isotopes of each element. If you're dealing with a specific isotopic composition, you'll need to use the precise atomic masses of those isotopes.
-
Polyatomic Ions: When calculating the molar mass of a compound containing polyatomic ions (like sulfate or phosphate), remember to consider the total mass of the ion as a single unit.
-
Hydrates: Compounds can exist as hydrates, meaning they incorporate water molecules into their crystal structure. The water molecules must be included when determining the molar mass.
-
Empirical vs. Molecular Formulas: If only the empirical formula is given, you'll need additional information (like the molar mass of the compound) to determine the molecular formula before calculating the number of molecules.
Conclusion
Determining the number of molecules in a compound is a fundamental skill in chemistry, vital for various applications. By understanding the concepts of moles, Avogadro's number, and molar mass, and carefully following the steps outlined in this guide, you can accurately calculate the number of molecules present in a given amount of substance. Remember to pay attention to units, significant figures, and any specific considerations related to the compound's characteristics, to ensure the precision and accuracy of your results. Mastering this skill will strengthen your foundation in chemistry and pave the way for more complex calculations and analyses.
Latest Posts
Latest Posts
-
Explain One Major Difference Between Purines And Pyrimidines
Mar 31, 2025
-
What Percent Is 1 Out Of 20
Mar 31, 2025
-
What Is 6 To The Zeroth Power
Mar 31, 2025
-
Which Intermolecular Force Is The Weakest
Mar 31, 2025
-
How To Calculate Molar Mass Of A Gas
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Number Of Molecules In A Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
