How To Find The Neutrons In The Periodic Table
listenit
Mar 22, 2025 · 5 min read
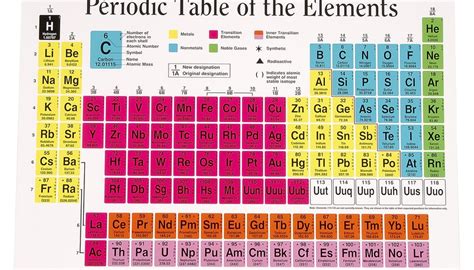
Table of Contents
How to Find (and Understand) Neutrons in the Periodic Table
The periodic table is a cornerstone of chemistry, organizing elements based on their atomic structure and properties. While it directly displays atomic number (protons) and often atomic mass, it doesn't explicitly list the number of neutrons. This might seem confusing at first, but understanding the relationship between protons, neutrons, and atomic mass allows you to indirectly determine the number of neutrons in an element's atom. This article will delve into the details of finding neutron numbers, explaining the concepts involved and providing practical examples.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we tackle the periodic table, let's refresh our understanding of atomic structure. An atom consists of three fundamental particles:
-
Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and its identity. For example, all hydrogen atoms have one proton, all helium atoms have two, and so on.
-
Neutrons: Neutrally charged particles also residing in the atom's nucleus. Unlike protons, the number of neutrons in an element can vary, leading to isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons usually equals the number of protons in a neutral atom, ensuring an overall neutral charge.
The Periodic Table and Atomic Number
The periodic table is arranged by atomic number, which is the number of protons in an atom's nucleus. This number is typically displayed above the element's symbol. For instance, hydrogen (H) has an atomic number of 1, helium (He) has 2, lithium (Li) has 3, and so on. This is the key piece of information we need to begin calculating the number of neutrons.
Isotopes and Atomic Mass: The Neutron's Hidden Clue
The periodic table usually lists an average atomic mass for each element. This average is weighted based on the abundance of different isotopes in nature. An isotope is an atom of the same element (same number of protons) but with a different number of neutrons. This difference in neutron number affects the atom's mass.
For example, carbon (C) has an atomic number of 6 (6 protons). Its average atomic mass is approximately 12.011 amu (atomic mass units). However, carbon exists in nature primarily as two isotopes: Carbon-12 (⁶C) with 6 neutrons and Carbon-13 (¹³C) with 7 neutrons. The average atomic mass of 12.011 reflects the relative abundance of these isotopes.
Calculating the Number of Neutrons: A Step-by-Step Guide
To determine the number of neutrons in a specific isotope, follow these steps:
-
Identify the atomic number (Z): This is found above the element's symbol on the periodic table.
-
Identify the mass number (A): This represents the total number of protons and neutrons in the nucleus of a specific isotope. It's often written as a superscript before the element's symbol (e.g., ²³⁸U). If only the average atomic mass is provided in the periodic table, you'll need to specify the isotope you're interested in. Note that the average atomic mass is a decimal number, reflecting the mixture of isotopes.
-
Calculate the number of neutrons (N): The number of neutrons is simply the difference between the mass number (A) and the atomic number (Z). The formula is:
N = A - Z
Example 1: Carbon-12 (¹²C)
-
Atomic number (Z) of Carbon = 6
-
Mass number (A) of Carbon-12 = 12
-
Number of neutrons (N) = 12 - 6 = 6 neutrons
Example 2: Uranium-238 (²³⁸U)
-
Atomic number (Z) of Uranium = 92 (from the periodic table)
-
Mass number (A) of Uranium-238 = 238
-
Number of neutrons (N) = 238 - 92 = 146 neutrons
Example 3: Using Average Atomic Mass (Approximation)
Let's say we want to estimate the number of neutrons in a typical chlorine atom. The periodic table shows chlorine (Cl) with an atomic number of 17 and an average atomic mass of approximately 35.45 amu. Because this is an average, we cannot determine the precise number of neutrons. However, we can approximate:
-
Atomic number (Z) of Chlorine = 17
-
Approximate Mass number (A) = 35 (rounding the average atomic mass)
-
Approximate number of neutrons (N) = 35 - 17 = 18 neutrons
Important Note: This approximation only gives an average neutron count. Chlorine has two main isotopes, Cl-35 and Cl-37, each with a different number of neutrons.
Beyond the Basics: Nuclear Stability and Isotope Abundance
The number of neutrons significantly impacts an atom's stability. Isotopes with unstable nuclei undergo radioactive decay, emitting particles and energy to reach a more stable state. The abundance of isotopes in nature reflects this stability. More stable isotopes are generally more abundant. The average atomic mass on the periodic table is a weighted average, taking into account the abundance of each isotope.
Advanced Applications: Nuclear Chemistry and Physics
Understanding the relationship between protons, neutrons, and isotopes is crucial in various scientific fields:
-
Nuclear Chemistry: This branch of chemistry focuses on the behavior of atomic nuclei, including radioactive decay and nuclear reactions. Knowledge of isotopes and neutron numbers is essential for understanding nuclear processes.
-
Nuclear Physics: This field explores the fundamental properties and interactions of atomic nuclei. Neutron behavior plays a crucial role in nuclear reactions and fission processes.
-
Medical Applications: Radioactive isotopes, often created by altering neutron numbers, are used extensively in medical imaging (PET scans) and radiotherapy for cancer treatment.
Conclusion: Mastering Neutron Calculation and Its Significance
While the periodic table doesn't explicitly state the number of neutrons for each element, understanding atomic structure and isotopic variations allows for the calculation of neutron numbers. This understanding is fundamental to various scientific fields, from chemistry and physics to medicine. By mastering the simple formula (N = A - Z), you gain a deeper appreciation for the complexity and richness of the atomic world and how the periodic table provides valuable insights into the fundamental building blocks of matter. Remember that the average atomic mass on the periodic table offers only an approximation when calculating neutrons. For precise neutron counts, you must identify the specific isotope under consideration. The information presented here serves as a solid foundation for further exploration of nuclear chemistry and physics.
Latest Posts
Latest Posts
-
What Is The 4th Root Of 16
Mar 24, 2025
-
What Is 3 5 As A Decimal
Mar 24, 2025
-
1 3 Divided By 6 In Fraction Form
Mar 24, 2025
-
Number Of Valence Electrons In Helium
Mar 24, 2025
-
Least Common Multiple Of 8 And 16
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Neutrons In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
