Number Of Valence Electrons In Helium
listenit
Mar 24, 2025 · 5 min read
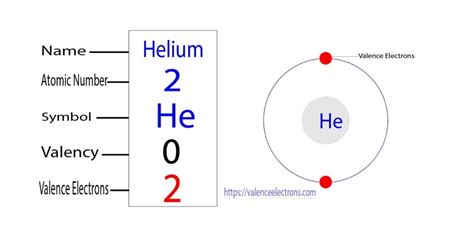
Table of Contents
The Enigmatic Helium: Delving Deep into its Valence Electrons
Helium, the second element on the periodic table, is a fascinating subject for study, particularly regarding its valence electrons. Unlike many other elements, helium's valence electron configuration plays a crucial role in its unique properties, its inert nature, and its significant applications. This comprehensive exploration will delve deep into the number of valence electrons in helium, exploring its electronic structure, chemical behavior, and the implications of its electron configuration.
Understanding Valence Electrons: The Key to Reactivity
Before diving into helium's specifics, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell or energy level of an atom. These electrons are the primary players in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons often dictates the number of bonds an atom can make, influencing its chemical properties. Elements with full valence shells, like the noble gases, are exceptionally stable and unreactive.
Helium's Electronic Structure: A Closer Look
Helium possesses an atomic number of 2, meaning it has two protons and two electrons in a neutral atom. Its electronic configuration is remarkably simple: 1s². This notation indicates that both electrons occupy the lowest energy level (n=1), specifically the s subshell. The s subshell can hold a maximum of two electrons, and in helium's case, it's completely filled. This complete valence shell is the key to understanding helium's unique characteristics.
The Significance of the Filled 1s Orbital
The completely filled 1s orbital is the cornerstone of helium's inertness. According to the octet rule, atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons (or two for the first shell). Helium, with its two electrons filling the first shell, already satisfies this rule. This complete valence shell makes it exceptionally stable and reluctant to participate in chemical reactions.
Why Helium Only Has Two Valence Electrons
Unlike elements in higher periods, helium's valence shell is the first shell (n=1). This first energy level only consists of the 1s orbital, which can accommodate a maximum of two electrons. Therefore, helium's two electrons completely fill its valence shell, resulting in its chemical inertness. Higher periods have more complex shell structures with multiple subshells (s, p, d, f), accommodating more electrons in their valence shells and allowing for greater reactivity and varied chemical behavior.
Comparing Helium's Valence Electrons to Other Noble Gases
While other noble gases like neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) also have full valence shells and are inert, their electron configurations differ. These elements have more electron shells and subshells, making their electron configurations more complex than helium's simple 1s². However, the common characteristic among all noble gases, including helium, is the presence of a completely filled outermost electron shell, resulting in exceptional stability and low reactivity.
The Implications of Helium's Filled Valence Shell
The filled valence shell of helium has profound consequences on its physical and chemical properties:
-
Inertness: Helium's reluctance to form chemical bonds makes it chemically inert. This property is crucial in its many applications.
-
Low Boiling Point: The weak interatomic forces between helium atoms, a consequence of its full valence shell and lack of chemical reactivity, result in an extremely low boiling point, making it a liquid only at extremely low temperatures.
-
Monatomic Nature: Helium exists as a monatomic gas, meaning it exists as individual atoms rather than forming molecules. This is directly related to its filled valence shell and its inability to form stable chemical bonds.
Applications of Helium: Leveraging its Unique Properties
Helium's unique properties, stemming from its filled valence shell, have made it invaluable in various applications:
-
Cryogenics: Helium's extremely low boiling point makes it ideal for use as a cryogenic refrigerant, cooling superconducting magnets in MRI machines and other scientific instruments.
-
Welding and Leak Detection: Helium's inertness and low density make it an excellent shielding gas in welding processes and a suitable tracer gas for leak detection.
-
Balloons and Airships: Helium's low density and buoyancy, coupled with its inertness, make it a safe and effective lifting gas for balloons and airships.
-
Scientific Research: Helium's inertness and other unique properties make it indispensable in various scientific research areas, including spectroscopy and chromatography.
Misconceptions about Helium's Valence Electrons
It's important to address common misconceptions regarding helium's valence electrons:
-
Helium doesn't "want" to gain or lose electrons: The notion that atoms "want" to achieve a stable configuration is an oversimplification. Helium's stability is a consequence of its filled valence shell, not a desire.
-
Helium's inertness is absolute: While helium is highly inert, extremely high pressures and temperatures can force it to participate in some chemical reactions, although these are rare and exceptional.
-
The octet rule isn't universally applicable: While the octet rule provides a useful guideline for many elements, it doesn't apply to all elements, particularly those in the first and second periods. Helium is a prime example, demonstrating that stability can be achieved with two valence electrons in the first shell.
Conclusion: The Importance of Helium's Electronic Structure
Helium's electronic structure, with its two valence electrons filling the 1s orbital, is fundamental to its unique properties and wide-ranging applications. Its filled valence shell results in its remarkable inertness, low boiling point, and monatomic nature. Understanding the number of valence electrons in helium is crucial to appreciating its significance in science, technology, and industry. Further research into helium's behavior under extreme conditions continues to provide valuable insights into its fundamental properties and the intricacies of electronic structure. From cryogenic applications to scientific instrumentation, helium's unique characteristics, directly linked to its filled valence shell, continue to shape our technological landscape. The seemingly simple element, helium, holds profound scientific significance, highlighting the importance of understanding basic atomic structure and its consequences on the macroscopic world. The study of helium serves as a powerful illustration of the fundamental principles governing chemical behavior and the power of a completely filled valence shell.
Latest Posts
Latest Posts
-
Is Rotting Wood A Chemical Change
Mar 25, 2025
-
How Do Humans Impact The Phosphorus Cycle
Mar 25, 2025
-
Where Does Dna Replication Take Place In Eukaryotic Cells
Mar 25, 2025
-
Through Which Medium Will Sound Travel Most Rapidly
Mar 25, 2025
-
Whats The Square Root Of 96
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Helium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
