How To Find Mass Of Excess Reactant
listenit
Mar 18, 2025 · 5 min read
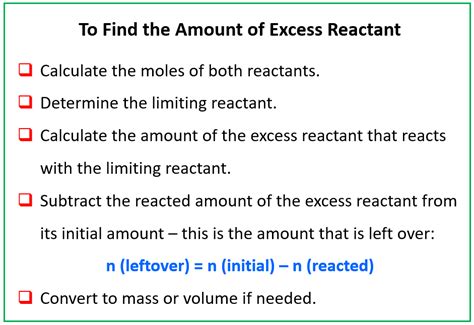
Table of Contents
How to Find the Mass of Excess Reactant: A Comprehensive Guide
Determining the mass of the excess reactant is a crucial step in many stoichiometry problems. Understanding this process is vital for chemists, students, and anyone working with chemical reactions. This comprehensive guide will walk you through the process step-by-step, explaining the underlying concepts and providing practical examples to solidify your understanding.
Understanding Stoichiometry and Limiting Reactants
Before diving into calculating the mass of the excess reactant, it's essential to grasp the fundamental concepts of stoichiometry and limiting reactants.
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It's based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. Therefore, the total mass of the reactants must equal the total mass of the products.
A limiting reactant (or limiting reagent) is the reactant that is completely consumed in a chemical reaction. Once the limiting reactant is used up, the reaction stops, regardless of how much of the other reactants remain. The other reactants are considered excess reactants.
Steps to Find the Mass of Excess Reactant
Finding the mass of the excess reactant involves several key steps:
1. Balance the Chemical Equation:
The first and most crucial step is to ensure the chemical equation representing the reaction is correctly balanced. This ensures the correct mole ratios between reactants and products are used in subsequent calculations. For example:
2H₂ + O₂ → 2H₂O
This equation shows that two moles of hydrogen (H₂) react with one mole of oxygen (O₂) to produce two moles of water (H₂O).
2. Convert Grams to Moles:
Next, convert the given masses of reactants into moles using their respective molar masses. The molar mass is the mass of one mole of a substance, usually expressed in grams per mole (g/mol). You can find molar masses on the periodic table or by calculating them from the atomic masses of the constituent elements.
-
Example: If you have 10 grams of hydrogen (H₂) and 20 grams of oxygen (O₂), you would use the molar masses of hydrogen (approximately 2 g/mol) and oxygen (approximately 32 g/mol) to convert to moles:
Moles of H₂ = 10 g / 2 g/mol = 5 moles Moles of O₂ = 20 g / 32 g/mol = 0.625 moles
3. Determine the Limiting Reactant:
Using the balanced chemical equation's mole ratios, determine which reactant is the limiting reactant. Compare the mole ratios of the reactants to the stoichiometric ratios in the balanced equation.
-
Example: From the balanced equation, we know that 2 moles of H₂ react with 1 mole of O₂. We have 5 moles of H₂ and 0.625 moles of O₂. To determine the limiting reactant, we can calculate how many moles of O₂ would be needed to react completely with the 5 moles of H₂:
Moles of O₂ needed = (5 moles H₂) * (1 mole O₂ / 2 moles H₂) = 2.5 moles O₂
Since we only have 0.625 moles of O₂, oxygen (O₂) is the limiting reactant. Hydrogen (H₂) is the excess reactant.
4. Calculate Moles of Excess Reactant Consumed:
Now, calculate how many moles of the excess reactant (hydrogen, in this case) react with the limiting reactant (oxygen). Use the mole ratio from the balanced equation.
-
Example: Since 1 mole of O₂ reacts with 2 moles of H₂, 0.625 moles of O₂ will react with:
Moles of H₂ consumed = (0.625 moles O₂) * (2 moles H₂ / 1 mole O₂) = 1.25 moles H₂
5. Calculate Moles of Excess Reactant Remaining:
Subtract the moles of excess reactant consumed from the initial moles of excess reactant to find the moles of excess reactant remaining.
-
Example: We started with 5 moles of H₂ and consumed 1.25 moles. Therefore:
Moles of H₂ remaining = 5 moles - 1.25 moles = 3.75 moles
6. Convert Moles to Grams:
Finally, convert the moles of excess reactant remaining back to grams using its molar mass.
-
Example: To find the mass of excess hydrogen remaining:
Mass of H₂ remaining = 3.75 moles * 2 g/mol = 7.5 g
Advanced Considerations and Complex Scenarios
While the steps above provide a general framework, real-world scenarios can be more complex. Let's explore some advanced considerations:
1. Reactions with More Than Two Reactants:
The process remains similar, but you'll need to compare the mole ratios of all reactants to identify the limiting reactant. Systematically determine how much of each reactant is needed to consume all others. The reactant that requires the least amount of all other reactants to be completely consumed is the limiting reactant.
2. Reactions with Percent Yield:
If the reaction doesn't proceed to 100% completion (a situation described by the percent yield), you'll need to adjust your calculations. First, calculate the theoretical yield based on the limiting reactant. Then, multiply the theoretical yield by the percent yield to get the actual yield. The amount of excess reactant consumed will also be proportionally reduced.
3. Reactions with Multiple Steps:
For reactions involving multiple steps, you will need to calculate the amount of product from each step and use that to calculate the amounts in subsequent steps. The limiting reactant in one step may produce a product which acts as a reactant in the next.
4. Impure Reactants:
If your reactants are not 100% pure, you'll need to account for the impurities. Calculate the amount of pure reactant available, then proceed with the stoichiometry calculations using this adjusted amount.
Practical Applications and Importance
The ability to accurately determine the mass of the excess reactant has significant practical applications across various fields:
- Chemical Engineering: Optimizing reaction conditions and maximizing product yield require precise knowledge of reactant amounts and ratios.
- Pharmaceutical Industry: In drug synthesis, precise stoichiometry ensures the desired product is produced without unwanted byproducts.
- Environmental Science: Understanding reaction stoichiometry helps model environmental processes and predict pollutant concentrations.
- Materials Science: The creation of new materials often involves precisely controlled chemical reactions, where knowledge of excess reactant amounts is crucial.
Conclusion
Finding the mass of the excess reactant is a fundamental skill in stoichiometry, with far-reaching applications in many scientific and industrial fields. By carefully following the steps outlined above and understanding the underlying concepts, you can confidently tackle stoichiometry problems and confidently navigate the world of chemical reactions. Remember to always double-check your calculations and ensure you are using the correct units throughout the process. With practice, these calculations will become second nature.
Latest Posts
Latest Posts
-
A Chef Is Going To Use A Mixture
Mar 19, 2025
-
How Many Feet In 120 In
Mar 19, 2025
-
What Percent Is 33 Out Of 40
Mar 19, 2025
-
Does Hcl Have Dipole Dipole Forces
Mar 19, 2025
-
Where Is The T Bone In A Cow
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Find Mass Of Excess Reactant . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
