Does Hcl Have Dipole Dipole Forces
listenit
Mar 19, 2025 · 5 min read
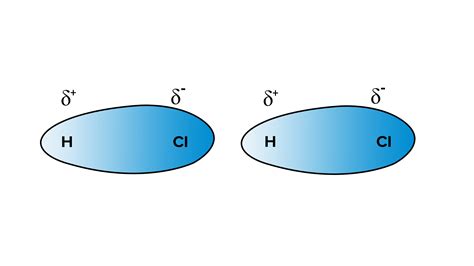
Table of Contents
Does HCL Have Dipole-Dipole Forces? Understanding Intermolecular Forces in Hydrogen Chloride
Hydrogen chloride (HCl), a simple yet crucial molecule, presents a fascinating case study in understanding intermolecular forces. While the presence of some intermolecular forces is evident, the specifics, particularly the role of dipole-dipole forces, require a closer examination. This article will delve deep into the intermolecular forces present in HCl, explaining the concept of dipole-dipole interactions and definitively answering the question: Does HCl have dipole-dipole forces? The answer, as we'll see, is a resounding yes, but with important nuances to consider.
Understanding Intermolecular Forces
Before focusing on HCl, let's establish a solid foundation by defining intermolecular forces (IMFs). These are the forces of attraction or repulsion between molecules, distinct from the intramolecular forces (bonds) within a molecule. IMFs are responsible for many of the physical properties of substances, including boiling point, melting point, viscosity, and surface tension. The strength of these forces dictates how easily molecules can move past each other, influencing the state of matter (solid, liquid, or gas).
Several types of IMFs exist, each varying in strength:
-
London Dispersion Forces (LDFs): Present in all molecules, LDFs arise from temporary fluctuations in electron distribution, creating instantaneous dipoles. These are the weakest type of IMF. The strength of LDFs increases with the size and shape of the molecule.
-
Dipole-Dipole Forces: These forces exist between polar molecules, molecules with a permanent dipole moment. The positive end of one polar molecule attracts the negative end of another, resulting in a stronger attraction than LDFs.
-
Hydrogen Bonding: A special type of dipole-dipole interaction, hydrogen bonding occurs when a hydrogen atom is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule. This is the strongest type of dipole-dipole interaction.
The Polar Nature of HCl
To understand the intermolecular forces in HCl, we must first consider its molecular structure and bonding. HCl is a diatomic molecule formed by a covalent bond between a hydrogen atom and a chlorine atom. However, chlorine is significantly more electronegative than hydrogen. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. This difference in electronegativity leads to a polar covalent bond.
In the HCl molecule, the chlorine atom pulls the shared electrons closer to itself, resulting in a partial negative charge (δ-) on the chlorine atom and a partial positive charge (δ+) on the hydrogen atom. This uneven distribution of charge creates a permanent dipole moment, meaning the molecule has a positive and a negative end. This polarity is crucial for determining the types of intermolecular forces present.
Dipole-Dipole Forces in HCl: A Definitive Yes
Given the polar nature of the HCl molecule, the presence of dipole-dipole forces is unequivocal. The positive hydrogen end of one HCl molecule is attracted to the negative chlorine end of another HCl molecule. These attractions contribute significantly to the overall intermolecular forces holding HCl molecules together. This is why HCl exists as a gas at room temperature but can be liquefied under pressure – the dipole-dipole forces, while not as strong as hydrogen bonding, are still substantial enough to influence its physical state.
Comparing HCl's IMFs with other Molecules
It's helpful to compare HCl's intermolecular forces with those of other molecules. Consider:
-
HF (Hydrogen Fluoride): HF exhibits hydrogen bonding, a stronger type of dipole-dipole interaction, due to the exceptionally high electronegativity of fluorine. This leads to a higher boiling point for HF compared to HCl.
-
HBr (Hydrogen Bromide) and HI (Hydrogen Iodide): These molecules also possess dipole-dipole forces, but the difference in electronegativity between the hydrogen and halogen atoms is less pronounced than in HCl. This results in weaker dipole-dipole forces and lower boiling points compared to HCl.
-
Nonpolar Molecules: Molecules like methane (CH₄) or oxygen (O₂) lack a permanent dipole moment and rely solely on London Dispersion Forces for intermolecular interactions. These forces are considerably weaker, resulting in much lower boiling points.
The Role of London Dispersion Forces in HCl
While dipole-dipole forces are dominant in HCl, it's crucial to remember that London Dispersion Forces are always present. Even though they are weaker than dipole-dipole interactions in this case, LDFs still contribute to the overall intermolecular attraction between HCl molecules. The combined effect of dipole-dipole forces and LDFs determines the physical properties of HCl.
Factors Influencing the Strength of Dipole-Dipole Interactions in HCl
The strength of dipole-dipole interactions in HCl is influenced by several factors:
-
Magnitude of the dipole moment: A larger dipole moment indicates a stronger dipole-dipole interaction. The dipole moment of HCl is relatively significant compared to other molecules of similar size, contributing to its comparatively stronger intermolecular forces.
-
Molecular shape: The linear shape of HCl facilitates efficient alignment of dipoles, maximizing the attractive forces between molecules. More complex molecular shapes can hinder optimal alignment and reduce the effectiveness of dipole-dipole forces.
-
Temperature: Increased temperature provides molecules with more kinetic energy, which can overcome the attractive forces of dipole-dipole interactions. This explains why HCl is a gas at room temperature, but can be liquefied by lowering the temperature.
Experimental Evidence Supporting Dipole-Dipole Forces in HCl
The physical properties of HCl provide strong experimental evidence for the presence of dipole-dipole forces. Its relatively high boiling point (compared to nonpolar molecules of similar molecular weight) and its ability to dissolve in polar solvents are consistent with the existence of significant dipole-dipole interactions. Spectroscopic techniques can also provide evidence of the polar nature of the HCl molecule, further supporting the presence of these intermolecular forces.
Conclusion: Dipole-Dipole Forces are Key for HCl
In summary, HCl does indeed have dipole-dipole forces. The polar nature of the HCl molecule, stemming from the difference in electronegativity between hydrogen and chlorine, leads to a permanent dipole moment. These dipoles interact with each other, creating significant dipole-dipole attractions between HCl molecules. While London Dispersion Forces are also present, they are less significant compared to the dipole-dipole interactions. The combined effects of these intermolecular forces determine the physical properties of HCl, such as its boiling point and solubility. Understanding these intermolecular forces is crucial for comprehending the behavior and applications of HCl in various scientific and industrial contexts. The presence of these forces highlights the importance of considering molecular polarity and electronegativity differences when predicting and explaining the properties of molecules.
Latest Posts
Latest Posts
-
Do Protists Make Their Own Food
May 09, 2025
-
38 Out Of 60 As A Percentage
May 09, 2025
-
How Many Ounces Are In 1 05 Qt
May 09, 2025
-
How To Determine Most Stable Chair Conformation
May 09, 2025
-
A Pair Of Pants With A Marked Price Of 35 00
May 09, 2025
Related Post
Thank you for visiting our website which covers about Does Hcl Have Dipole Dipole Forces . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.