How To Calculate The Ph At The Equivalence Point
listenit
Apr 01, 2025 · 6 min read
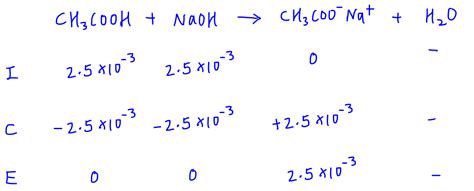
Table of Contents
How to Calculate the pH at the Equivalence Point
Determining the pH at the equivalence point of a titration is crucial for understanding the reaction's stoichiometry and selecting appropriate indicators. The equivalence point represents the theoretical point where the moles of acid and base are stoichiometrically equal, resulting in complete neutralization. However, calculating the exact pH at this point can be more complex than simply assuming a neutral pH of 7. The pH at the equivalence point depends significantly on the nature of the acid and base involved – strong/strong, strong/weak, or weak/weak. Let's delve into the methods for calculating the pH at the equivalence point for each scenario.
Strong Acid-Strong Base Titration
This is the simplest case. When a strong acid (like HCl) is titrated with a strong base (like NaOH), the equivalence point results in a neutral solution (pH 7) at 25°C. This is because the conjugate acid and base formed are extremely weak and have negligible impact on the solution's pH.
Calculation:
At the equivalence point:
- Moles of acid = Moles of base
Since both are strong, they completely dissociate. Therefore, calculating the pH is straightforward:
- Determine the moles of acid and base: Use the initial concentration and volume of the acid (or base) to find the moles.
- Calculate the total volume: Add the volumes of the acid and base used at the equivalence point.
- Calculate the concentration of the salt: The concentration of the salt formed is the moles of acid (or base) divided by the total volume.
- Calculate the pH: For a strong acid-strong base titration at the equivalence point, the pH is 7.0 (at 25°C). Any deviation from 7 can be attributed to experimental error or changes in temperature.
Example:
25 mL of 0.1 M HCl is titrated with 0.1 M NaOH. At the equivalence point, 25 mL of NaOH has been added. The pH will be 7.0.
Strong Acid-Weak Base Titration
The pH at the equivalence point of a strong acid-weak base titration is always less than 7 (acidic). This is because the conjugate acid of the weak base is formed, which can donate protons, lowering the pH.
Calculation:
- Determine the moles of acid and base: Use initial concentration and volume to find moles at the equivalence point.
- Calculate the concentration of the conjugate acid: The moles of conjugate acid are equal to the initial moles of the weak base. Divide by the total volume.
- Calculate the Ka of the conjugate acid: Use the relationship Ka * Kb = Kw (where Kw is the ion product constant of water, 1.0 x 10⁻¹⁴ at 25°C) to find Ka.
- Use the ICE table (Initial, Change, Equilibrium): Set up an ICE table to determine the equilibrium concentrations of the conjugate acid, its ions, and H⁺ ions.
- Solve for [H⁺]: Use the equilibrium expression for the conjugate acid's dissociation (Ka = [H⁺][A⁻]/[HA], where HA is the conjugate acid). This usually involves solving a quadratic equation. If Ka is very small, you can often simplify the calculation by neglecting the [H⁺] added to the initial concentration of the conjugate acid.
- Calculate the pH: pH = -log₁₀[H⁺]
Example:
Let's assume 25 mL of 0.1 M HCl is titrated with 25 mL of 0.1 M NH₃ (Kb = 1.8 x 10⁻⁵).
- Calculate Kb of NH4+ using Ka * Kb = Kw = 1 x 10^-14
- Calculate the concentration of NH₄⁺ (conjugate acid) after neutralization
- Use the ICE table and Ka to determine the H+ concentration and subsequently calculate the pH.
Weak Acid-Strong Base Titration
In this case, the pH at the equivalence point is greater than 7 (basic) due to the formation of the conjugate base of the weak acid. This conjugate base will accept protons from water, increasing the hydroxide ion concentration and raising the pH.
Calculation:
- Determine the moles of acid and base: Similar to previous examples.
- Calculate the concentration of the conjugate base: This is equal to the initial moles of the weak acid, divided by the total volume.
- Calculate the Kb of the conjugate base: Use the relationship Ka * Kb = Kw to find Kb from the known Ka of the weak acid.
- Use the ICE table: Set up an ICE table for the conjugate base's reaction with water (A⁻ + H₂O ⇌ HA + OH⁻).
- Solve for [OH⁻]: Use the equilibrium expression for the conjugate base's reaction (Kb = [HA][OH⁻]/[A⁻]). This might require solving a quadratic equation. Similar to the previous example, you can approximate the value if Kb is very small.
- Calculate the pOH: pOH = -log₁₀[OH⁻]
- Calculate the pH: pH = 14 - pOH
Example:
If 25 mL of 0.1 M acetic acid (CH₃COOH, Ka = 1.8 x 10⁻⁵) is titrated with 25 mL of 0.1 M NaOH.
- Calculate Kb of CH3COO- (conjugate base) using Ka * Kb = Kw = 1 x 10^-14
- Calculate the concentration of CH₃COO⁻ after neutralization
- Use the ICE table and Kb to determine the OH- concentration
- Calculate pOH and subsequently, the pH.
Weak Acid-Weak Base Titration
This is the most complex scenario. The pH at the equivalence point depends on the relative strengths of the weak acid and weak base. A simple approximation is often insufficient. The pH will be approximately 7 if the Ka and Kb values are very similar; otherwise, it will deviate from neutrality and can't be easily predicted without using a more sophisticated calculation.
Calculation:
This requires a more advanced approach, often involving:
- Considering both equilibrium reactions: The conjugate acid and base both contribute to the pH.
- Solving a system of simultaneous equations: The equilibrium expressions for both the conjugate acid and base must be solved simultaneously, which will lead to a more complicated equilibrium calculation.
- Using iterative methods or software: For accurate pH calculation, numerical methods or specialized chemical equilibrium software is often necessary. These calculations are often quite complex and not suited to manual calculation without sophisticated software.
Factors Affecting pH at the Equivalence Point
Several factors influence the pH at the equivalence point:
- Temperature: The Kw value of water changes with temperature, affecting the calculations involving Ka and Kb.
- Ionic strength: The presence of other ions in the solution can influence the activity coefficients of the species involved, slightly altering the equilibrium calculations.
- Experimental errors: Inaccurate measurements of volumes or concentrations will lead to a deviation from the theoretically calculated pH.
Practical Implications
Understanding how to calculate the pH at the equivalence point is vital in various applications:
- Titration analysis: Selecting a suitable indicator for a titration depends on the pH range over which the indicator changes color.
- Buffer solution preparation: Knowledge of equivalence point pH helps in determining the composition of effective buffer solutions.
- Chemical synthesis and analysis: Many chemical reactions are sensitive to pH, and understanding the equivalence point is crucial in controlling the reaction conditions.
Calculating the pH at the equivalence point requires careful consideration of the acid and base strengths involved. While strong acid-strong base titrations offer a straightforward calculation, titrations involving weak acids and/or bases necessitate more complex calculations. Using the methods outlined above, and potentially employing numerical methods or software for more complex scenarios, will provide accurate predictions of the equivalence point pH. Remember that even with meticulous calculations, slight deviations from theoretical predictions may occur due to experimental conditions.
Latest Posts
Latest Posts
-
How Many Electrons Are In Mercury
Apr 02, 2025
-
Whats The Square Root Of 33
Apr 02, 2025
-
What Happens To The Atoms During A Chemical Reaction
Apr 02, 2025
-
Is Delta H Positive Or Negative In An Endothermic Reaction
Apr 02, 2025
-
27 To The 1 3 Power
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate The Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
