How To Calculate Molarity From Titration
listenit
Mar 25, 2025 · 6 min read
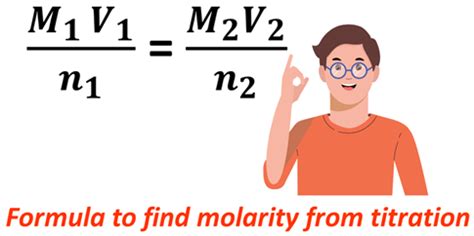
Table of Contents
How to Calculate Molarity from Titration: A Comprehensive Guide
Titration is a fundamental laboratory technique used in analytical chemistry to determine the concentration of an unknown solution, often referred to as the analyte. This process involves reacting the analyte with a solution of known concentration, called the titrant, until the reaction is complete. By carefully measuring the volume of titrant used, we can calculate the molarity (moles per liter) of the analyte. This guide provides a comprehensive walkthrough of how to calculate molarity from titration data, covering various scenarios and addressing potential challenges.
Understanding the Fundamentals: Molarity and Titration
Before delving into calculations, let's solidify our understanding of key concepts:
Molarity (M):
Molarity is a measure of concentration, expressed as the number of moles of solute per liter of solution. The formula is:
Molarity (M) = Moles of solute / Liters of solution
Titration:
Titration is a quantitative chemical analysis method where a solution of known concentration (titrant) is added gradually to a solution of unknown concentration (analyte) until the reaction between them is complete. This endpoint is often detected using an indicator, which changes color at or near the equivalence point.
Equivalence Point:
The equivalence point is the point in the titration where the moles of titrant added are stoichiometrically equivalent to the moles of analyte present. This is the theoretical point where the reaction is complete.
Endpoint:
The endpoint is the point in the titration where the indicator changes color, signaling the approximate completion of the reaction. Ideally, the endpoint should be very close to the equivalence point. However, a slight difference, known as the indicator error, might exist.
Types of Titrations and Relevant Calculations
Several types of titrations exist, each requiring slight adjustments in the calculation procedure. We will cover the most common types:
Acid-Base Titration:
This is the most common type of titration, involving the reaction of an acid with a base. The reaction typically involves the transfer of protons (H⁺). Consider the example of titrating a strong acid (like HCl) with a strong base (like NaOH):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Calculating Molarity:
-
Record data: Note the volume of the titrant (NaOH) used to reach the endpoint, its molarity (M<sub>NaOH</sub>), and the volume of the analyte (HCl) being titrated (V<sub>HCl</sub>).
-
Determine moles of titrant: Use the molarity and volume of the NaOH solution:
Moles of NaOH = M<sub>NaOH</sub> × V<sub>NaOH</sub> (in Liters)
-
Determine moles of analyte: Based on the stoichiometry of the balanced chemical equation (1:1 in this case), the moles of HCl are equal to the moles of NaOH at the equivalence point:
Moles of HCl = Moles of NaOH
-
Calculate the molarity of the analyte: Use the moles of HCl and the volume of the HCl solution:
M<sub>HCl</sub> = Moles of HCl / V<sub>HCl</sub> (in Liters)
Example: 25.00 mL of 0.100 M NaOH is used to titrate 20.00 mL of HCl solution. What is the molarity of the HCl solution?
Moles of NaOH = 0.100 M × (25.00 mL / 1000 mL/L) = 0.00250 moles Moles of HCl = 0.00250 moles M<sub>HCl</sub> = 0.00250 moles / (20.00 mL / 1000 mL/L) = 0.125 M
Redox Titration:
Redox titrations involve the transfer of electrons between the titrant and the analyte. These reactions often involve oxidation and reduction half-reactions. The calculations are similar to acid-base titrations, but the stoichiometry might be different, depending on the balanced redox equation.
Example: Titration of an iron(II) solution with potassium permanganate (KMnO₄). The balanced equation would dictate the mole ratio between the reactants, which is crucial for accurate molarity calculation.
Complexometric Titration:
This type of titration involves the formation of a complex between the titrant and the analyte. EDTA (ethylenediaminetetraacetic acid) is a common chelating agent used in complexometric titrations. The calculations are similar, but the stoichiometry of the complex formation reaction must be considered.
Addressing Common Challenges and Errors
Several factors can influence the accuracy of titration results. It's crucial to be aware of these potential sources of error:
-
Indicator Error: The endpoint might not precisely coincide with the equivalence point. This difference is the indicator error. Selecting an appropriate indicator and practicing good titration technique minimizes this error.
-
Improperly Calibrated Equipment: Using inaccurate volumetric glassware (burets, pipettes) can lead to significant errors. Always ensure your glassware is properly calibrated.
-
Impurities in Solutions: The presence of impurities in either the titrant or analyte solution can affect the results. Using high-purity chemicals and properly cleaning glassware are crucial.
-
Temperature Fluctuations: Temperature changes can affect the reaction rate and equilibrium, impacting the accuracy of the titration. Control the temperature as consistently as possible.
-
Incomplete Reaction: Failure to allow sufficient time for the reaction to reach completion will lead to an inaccurate determination of the endpoint.
Advanced Titration Techniques and Calculations
Some titrations involve more complex scenarios that require additional considerations:
Back Titration:
In back titrations, an excess of titrant is added to the analyte, and then the remaining titrant is titrated with a second standard solution. This technique is useful when the reaction between the analyte and titrant is slow or when the analyte is not easily dissolved.
The calculation involves determining the moles of titrant initially added and subtracting the moles of titrant remaining after the back titration. The difference represents the moles that reacted with the analyte.
Titration Curves:
Titration curves plot the pH (or another relevant property) of the solution against the volume of titrant added. Analyzing the titration curve helps identify the equivalence point more accurately. This is particularly valuable for weak acid-strong base or weak base-strong acid titrations, where the equivalence point doesn't necessarily correspond to a pH of 7.
Practical Tips for Accurate Titration and Calculation
-
Practice Proper Technique: Develop good laboratory skills, including accurate measuring, careful addition of titrant, and precise endpoint detection.
-
Use Appropriate Indicators: Select indicators with a color change close to the expected pH at the equivalence point.
-
Repeat the Titration: Performing multiple titrations and calculating the average molarity improves the accuracy and precision of the results.
-
Analyze Data Critically: Evaluate your data for outliers and potential sources of error. Consider the precision and accuracy of your results.
-
Proper Data Recording: Keep a detailed record of all your experimental data, including volumes, molarity of standard solutions, and observations during the titration.
Conclusion
Calculating molarity from titration data is a critical skill in analytical chemistry. Understanding the principles of titration, mastering the calculations, and being aware of potential errors are essential for obtaining reliable and accurate results. By carefully following the steps outlined in this guide and practicing good laboratory technique, you can confidently determine the concentration of unknown solutions using titration. Remember to always double-check your calculations and consider the limitations of the method to achieve high accuracy and reliability in your analyses. The more experience you gain in performing and interpreting titrations, the better you’ll become at obtaining and interpreting the results.
Latest Posts
Latest Posts
-
Classify These Orbital Descriptions By Type
Mar 28, 2025
-
What Is Square Root Of 625
Mar 28, 2025
-
Which Statement Explains One Way That Minerals Form
Mar 28, 2025
-
What Is 6 11 As A Decimal
Mar 28, 2025
-
A B C Solve For B
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Molarity From Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
