Classify These Orbital Descriptions By Type
listenit
Mar 28, 2025 · 6 min read
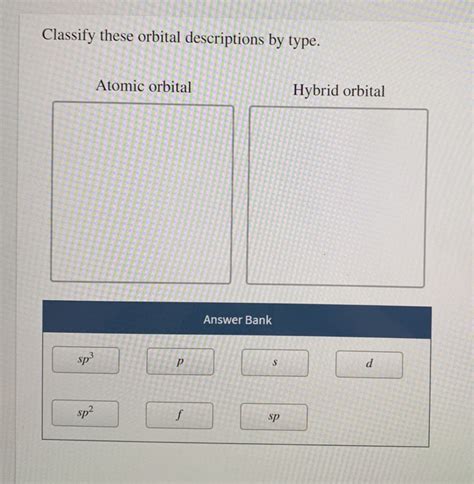
Table of Contents
Classify These Orbital Descriptions by Type: A Comprehensive Guide to Atomic Orbitals
Understanding atomic orbitals is fundamental to grasping the behavior of atoms and molecules. This article provides a comprehensive guide to classifying orbital descriptions based on their quantum numbers and shapes. We'll explore the different types of orbitals, their characteristics, and how to identify them based on their descriptions. This guide is designed to be both informative and accessible, catering to students and enthusiasts alike.
Understanding Quantum Numbers
Before diving into orbital classification, let's review the four quantum numbers that define an electron's state within an atom:
-
Principal Quantum Number (n): This number determines the energy level of the electron and the size of the orbital. It can be any positive integer (1, 2, 3...). Higher values of n indicate higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This number defines the shape of the orbital and its angular momentum. It can range from 0 to n - 1. Each value of l corresponds to a specific subshell:
- l = 0: s orbital (spherical)
- l = 1: p orbital (dumbbell-shaped)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. It can range from -l to +l, including 0. For example, a p subshell (l = 1) has three orbitals with ml = -1, 0, and +1, oriented along the x, y, and z axes, respectively.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum (spin) of the electron. It can have only two values: +1/2 (spin up) or -1/2 (spin down).
Classifying Orbital Descriptions
Now let's analyze how to classify orbital descriptions based on these quantum numbers. We'll look at several examples, demonstrating the process of identifying the type of orbital.
Example 1: n=2, l=1, ml=0, ms=+1/2
This description specifies:
- n=2: The electron is in the second principal energy level.
- l=1: The orbital is a p orbital (dumbbell shape).
- ml=0: The p orbital is oriented along the z-axis (pz orbital).
- ms=+1/2: The electron has a spin up.
Therefore, this describes a 2pz orbital with a spin-up electron.
Example 2: n=3, l=0, ml=0, ms=-1/2
This description tells us:
- n=3: The electron is in the third principal energy level.
- l=0: The orbital is an s orbital (spherical shape).
- ml=0: For s orbitals, ml is always 0 as there's only one orientation.
- ms=-1/2: The electron has a spin down.
This describes a 3s orbital with a spin-down electron.
Example 3: n=4, l=2, ml=+2, ms=+1/2
Here we have:
- n=4: Fourth principal energy level.
- l=2: A d orbital (more complex shapes).
- ml=+2: This specifies one of the five possible orientations of a d orbital.
- ms=+1/2: Spin up electron.
This represents a 4d orbital with a specific orientation and a spin-up electron. Note that without a visualization, the exact spatial orientation of the d orbital cannot be precisely determined from only the ml value.
Example 4: n=1, l=0
This description is incomplete because it only provides the principal quantum number and the azimuthal quantum number. It tells us:
- n=1: First principal energy level.
- l=0: An s orbital.
This describes a 1s orbital, but the magnetic and spin quantum numbers are missing, meaning it's not fully specifying the electron's state.
Example 5: A spherical orbital with a node at the nucleus
This description focuses on the shape and nodal properties. The presence of a spherical shape and a node at the nucleus strongly suggests a 2s orbital. The 1s orbital is also spherical, but it does not have a node at the nucleus. The 2s orbital has a spherical node which is a region of zero electron density. Higher energy s orbitals also possess multiple radial nodes.
Example 6: A dumbbell-shaped orbital
This description indicates a p orbital. However, further information, such as the principal quantum number and the orientation (ml), is required for complete classification (e.g., 2px, 3py, etc.).
Visualizing Orbitals
Understanding the shapes of orbitals is crucial for visualizing electron distributions within atoms. While precise visualizations can be complex, we can simplify them:
-
s orbitals: These are spherically symmetric. The 1s orbital is a single sphere; higher-level s orbitals (2s, 3s, etc.) have additional radial nodes (regions of zero electron probability).
-
p orbitals: These have a dumbbell shape. There are three p orbitals (px, py, pz) oriented along the x, y, and z axes respectively.
-
d orbitals: These have more complex shapes. There are five d orbitals, with various orientations and nodal planes (regions of zero electron probability).
-
f orbitals: These have even more intricate shapes and are difficult to visualize easily.
Beyond Basic Classification
The above classifications cover basic atomic orbitals in isolated atoms. However, in molecules, the interaction between atoms leads to the formation of molecular orbitals, which are significantly more complex and involve linear combinations of atomic orbitals. Understanding the hybridization of atomic orbitals further complicates the classification process. Nevertheless, the fundamental principles of quantum numbers and orbital shapes remain important building blocks for understanding molecular orbitals and chemical bonding.
Advanced Considerations and Applications
The accurate classification of atomic orbitals is not merely an academic exercise. It has direct applications across several scientific fields:
-
Quantum Chemistry: Accurate orbital descriptions are the foundation of computational methods used to predict molecular properties and reactivity. Software packages utilize these principles to model complex chemical systems.
-
Spectroscopy: The transition of electrons between different atomic orbitals gives rise to characteristic absorption and emission spectra. Analyzing these spectra helps identify elements and understand their electronic structure.
-
Materials Science: Orbital classification is fundamental to understanding the electronic properties of materials. The arrangement and types of orbitals determine whether a material is a conductor, insulator, or semiconductor, influencing their applications in electronics and other technologies.
Conclusion
Classifying orbital descriptions by type requires a thorough understanding of quantum numbers. By carefully analyzing the provided quantum numbers (n, l, ml, ms) or characteristics such as the orbital shape and nodal properties, one can accurately identify the type of orbital and its electron occupancy. This knowledge is crucial not only for understanding atomic structure but also for advancements in various scientific and technological disciplines. This detailed guide provides a comprehensive framework for mastering this essential aspect of chemistry and physics. Remember to practice classifying orbital descriptions using various examples to solidify your understanding. The more you practice, the more proficient you will become in identifying and distinguishing between different types of atomic orbitals.
Latest Posts
Latest Posts
-
How To Find An Objects Acceleration
Mar 31, 2025
-
How Do Igneous Rocks Form Into Sedimentary Rocks
Mar 31, 2025
-
What Is The Sum Of A Heptagons Interior Angles
Mar 31, 2025
-
What Is The Inverse Of X 3
Mar 31, 2025
-
What Is The Gcf Of 42 And 24
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Classify These Orbital Descriptions By Type . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
