How To Calculate Enthalpy Of Neutralisation
listenit
Mar 31, 2025 · 6 min read
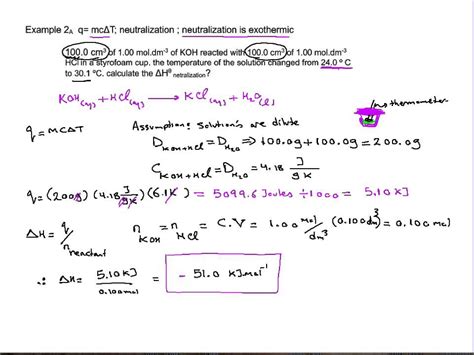
Table of Contents
How to Calculate the Enthalpy of Neutralization
Enthalpy of neutralization is a crucial concept in chemistry, representing the heat change occurring during an acid-base reaction. Understanding how to calculate it is essential for students and professionals alike. This comprehensive guide will walk you through the process, explaining the underlying principles and offering practical examples. We'll delve into different methods, address potential challenges, and provide tips for accurate calculations.
Understanding Enthalpy of Neutralization
Before diving into calculations, let's establish a firm understanding of the concept. Enthalpy of neutralization (ΔH<sub>n</sub>) is the enthalpy change when one mole of acid reacts completely with one mole of base under standard conditions (usually 298K and 1 atm). This reaction typically involves the formation of water and a salt. The reaction is exothermic, meaning heat is released, and therefore ΔH<sub>n</sub> is usually negative.
Types of Neutralization Reactions
The enthalpy of neutralization varies slightly depending on the strength of the acids and bases involved.
-
Strong Acid-Strong Base: Reactions between strong acids (like HCl, HNO₃, H₂SO₄) and strong bases (like NaOH, KOH) exhibit a relatively constant enthalpy of neutralization. This is because strong acids and bases dissociate completely in solution, resulting in a consistent reaction. The enthalpy change is approximately -57 kJ/mol.
-
Weak Acid-Strong Base or Strong Acid-Weak Base: In these scenarios, the enthalpy of neutralization will be less exothermic (a less negative value). This is due to the energy required to ionize the weak acid or base. Part of the heat released during the neutralization reaction is absorbed in the ionization process.
-
Weak Acid-Weak Base: Reactions between weak acids and weak bases are complex, and calculating the enthalpy of neutralization is considerably more challenging. The ionization of both the acid and the base significantly impacts the overall enthalpy change.
Methods for Calculating Enthalpy of Neutralization
The most common method to determine the enthalpy of neutralization is through calorimetry. This technique involves measuring the temperature change during the reaction to calculate the heat released or absorbed.
Calorimetric Method
This method relies on the principle of conservation of energy: the heat released by the reaction is absorbed by the solution and the calorimeter itself. Here's a step-by-step guide:
-
Experimental Setup: The reaction is carried out in a calorimeter, an insulated container designed to minimize heat exchange with the surroundings. The calorimeter typically contains a known volume of acid solution, and the base is added carefully. A thermometer is used to monitor the temperature change.
-
Temperature Measurement: The initial temperature (T₁) of the acid solution is recorded before the addition of the base. After the addition and thorough mixing, the final temperature (T₂) is recorded. It's crucial to ensure the reaction reaches thermal equilibrium before taking the final temperature reading.
-
Heat Capacity Determination: The heat capacity (C) of the calorimeter needs to be determined. This can be done using a calibration experiment (e.g., using a known heat source). This value represents the amount of heat required to raise the temperature of the calorimeter by 1°C.
-
Calculations: The heat released (q) by the neutralization reaction can be calculated using the formula:
q = - (m × c × ΔT + C × ΔT)
Where:
- q = heat released (in Joules)
- m = mass of the solution (in grams)
- c = specific heat capacity of the solution (usually assumed to be similar to water, 4.18 J/g°C)
- ΔT = change in temperature (T₂ - T₁)
- C = heat capacity of the calorimeter (in J/°C)
The negative sign indicates that the reaction is exothermic.
-
Moles Calculation: Determine the number of moles (n) of the limiting reactant (acid or base) involved in the reaction. This is usually determined using the concentration and volume of the solutions used.
-
Enthalpy Calculation: Finally, the enthalpy of neutralization (ΔH<sub>n</sub>) is calculated by dividing the heat released by the number of moles of the limiting reactant:
ΔH<sub>n</sub> = q / n
The result will be in Joules per mole (J/mol). It's often converted to kilojoules per mole (kJ/mol) for convenience.
Addressing Potential Errors
Accurate enthalpy of neutralization calculations rely on meticulous experimental techniques. Here are some potential sources of error and how to minimize them:
-
Heat Loss: Heat loss to the surroundings is a significant source of error. Using a well-insulated calorimeter and ensuring rapid mixing helps to minimize this.
-
Incomplete Reaction: Ensure that the reaction goes to completion. Using excess amounts of one reactant is advisable for complete neutralization.
-
Specific Heat Capacity: The assumption that the specific heat capacity of the solution is equal to that of water is an approximation. The specific heat capacity of the solution will vary depending on the concentrations of acid and base used. The use of a more accurate specific heat capacity will improve the results.
-
Heat of Dilution: The process of diluting the acid and base solution can also contribute to the heat change, so this needs to be taken into consideration.
Advanced Considerations
For more complex scenarios involving weak acids or bases, the calculation becomes significantly more intricate. More advanced techniques might be needed, such as:
-
Thermodynamic Data: Using standard enthalpy of formation data for the reactants and products.
-
Titration Curves: Precise measurements to determine the neutralization point and heat changes during the reaction.
-
Computer Modeling: Using computational techniques for a more precise and detailed analysis.
Example Calculation
Let's illustrate the calorimetric method with an example:
50.0 cm³ of 1.00 mol/dm³ hydrochloric acid (HCl) is reacted with 50.0 cm³ of 1.00 mol/dm³ sodium hydroxide (NaOH) in a calorimeter. The initial temperature was 20.0°C, and the final temperature was 26.5°C. The heat capacity of the calorimeter is 10.0 J/°C. Assume the specific heat capacity of the solution is 4.18 J/g°C and the density of the solution is 1.00 g/cm³.
-
Calculate the mass of the solution: Total volume = 100 cm³ = 100 g (since density = 1.00 g/cm³)
-
Calculate the temperature change (ΔT): ΔT = 26.5°C - 20.0°C = 6.5°C
-
Calculate the heat released (q):
q = - (100 g × 4.18 J/g°C × 6.5°C + 10.0 J/°C × 6.5°C) = -2820.5 J
-
Calculate the number of moles of the limiting reactant: Moles of HCl = (1.00 mol/dm³) × (0.050 dm³) = 0.050 mol. Moles of NaOH = (1.00 mol/dm³) × (0.050 dm³) = 0.050 mol. In this case, both reactants are in equal stoichiometric amounts.
-
Calculate the enthalpy of neutralization (ΔH<sub>n</sub>):
ΔH<sub>n</sub> = -2820.5 J / 0.050 mol = -56410 J/mol = -56.4 kJ/mol
This calculation provides an approximate value for the enthalpy of neutralization. The slight deviation from the theoretical -57 kJ/mol is likely due to experimental errors like heat loss.
Conclusion
Calculating the enthalpy of neutralization involves careful experimental procedures and precise calculations. While the basic calorimetric method provides a good approximation, addressing potential sources of error and considering the strengths of the acid and base are crucial for obtaining accurate results. Understanding the underlying principles and employing appropriate techniques will enhance your understanding of this fundamental chemical concept. Remember that precision is key in obtaining reliable data in chemistry experiments, and careful consideration of all factors will yield more accurate results. Further research into advanced techniques can provide more precise calculations for more complex neutralization reactions.
Latest Posts
Latest Posts
-
What Are Three Parts Of Atp Molecule
Apr 01, 2025
-
How Many Right Angles Does A Quadrilateral Have
Apr 01, 2025
-
Is Magnesium A Gas Liquid Or Solid
Apr 01, 2025
-
Where In The Chloroplast Do The Light Reactions Take Place
Apr 01, 2025
-
What Is All The Colors Combined
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Enthalpy Of Neutralisation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
