How Many Valence Electros In Hydrogen
listenit
Mar 17, 2025 · 5 min read
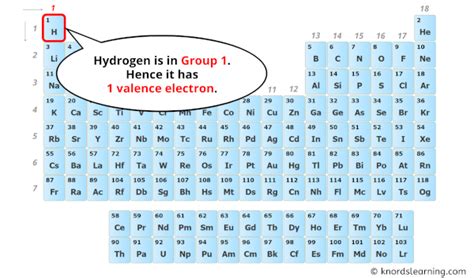
Table of Contents
How Many Valence Electrons Does Hydrogen Have? A Deep Dive into Atomic Structure
Hydrogen, the simplest and most abundant element in the universe, often serves as a foundational element in understanding basic chemistry principles. One of the most fundamental questions about hydrogen is: how many valence electrons does it possess? This seemingly simple question opens the door to a deeper exploration of atomic structure, electron configuration, and the chemical behavior of this crucial element.
Understanding Valence Electrons
Before delving into the specifics of hydrogen, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell or energy level of an atom. These electrons are the primary players in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons directly impacts an element's position on the periodic table and its chemical properties.
Atoms strive for stability, often achieved by having a full outermost electron shell. This principle is known as the octet rule, although it has exceptions, particularly for elements in the first and second rows of the periodic table. Atoms will gain, lose, or share electrons to attain this stable configuration, resulting in the formation of chemical bonds.
Hydrogen's Unique Position
Hydrogen occupies a unique position on the periodic table. While it's placed in Group 1 (alkali metals), its behavior differs significantly from other alkali metals. This difference stems from its simple atomic structure.
Hydrogen's Atomic Structure: One Proton, One Electron
A hydrogen atom contains only one proton in its nucleus and one electron orbiting the nucleus. This single electron resides in the first energy level (n=1), which can hold a maximum of two electrons. Because the first energy level is the outermost shell in hydrogen, this single electron is also its valence electron.
Therefore, hydrogen has one valence electron.
This single valence electron is responsible for hydrogen's chemical behavior. Hydrogen readily forms covalent bonds by sharing its electron with another atom to achieve a stable electron configuration similar to helium (1s²). It can also lose its electron to form a positively charged hydrogen ion (H+), although this is less common.
Hydrogen's Bonding Capabilities: Covalent and Ionic Bonds
Hydrogen's single valence electron dictates its bonding behavior. Let's examine the types of bonds it forms:
Covalent Bonding in Hydrogen
In covalent bonding, atoms share electrons to achieve a stable electron configuration. A classic example is the hydrogen molecule (H₂), where two hydrogen atoms share their single valence electrons to form a single covalent bond. Each hydrogen atom effectively achieves a full electron shell (similar to helium) by sharing the electrons. This bond is exceptionally strong and plays a vital role in many chemical processes.
Ionic Bonding Involving Hydrogen
Although less prevalent than covalent bonding, hydrogen can also participate in ionic bonding under certain circumstances. In this type of bond, one atom transfers one or more electrons to another atom. This transfer results in the formation of ions: a positively charged cation and a negatively charged anion.
In the case of hydrogen, it can lose its valence electron to form a positively charged hydrogen ion (H+), often called a proton due to the loss of its single electron. This ion then forms an ionic bond with a negatively charged ion. Examples of ionic compounds involving H+ include acids like hydrochloric acid (HCl) and sulfuric acid (H₂SO₄).
Hydrogen's Role in Chemical Reactions: A Versatile Element
The single valence electron of hydrogen makes it a highly versatile element, capable of participating in a wide range of chemical reactions. Its involvement in acid-base reactions, redox reactions, and organic chemistry is crucial.
Acid-Base Reactions
Hydrogen ions (H+) play a central role in acid-base chemistry, defining the acidity of a solution. The concentration of H+ ions determines the pH of a solution; a higher concentration of H+ leads to a lower pH (more acidic solution). Acids are substances that donate H+ ions, while bases accept H+ ions.
Redox Reactions
Hydrogen can also participate in redox (reduction-oxidation) reactions. In these reactions, electrons are transferred between atoms or molecules. Hydrogen can act as both an oxidizing agent (accepting electrons) and a reducing agent (donating electrons), depending on the reaction conditions. This ability contributes to its significant role in energy conversion processes.
Organic Chemistry: The Backbone of Life
Hydrogen is a fundamental constituent of organic molecules, the molecules that form the basis of life. It's part of hydrocarbons, carbohydrates, proteins, and nucleic acids. The C-H bond is ubiquitous in organic chemistry and plays a significant role in the stability and reactivity of organic compounds.
Beyond the Basics: Isotopes of Hydrogen and their Valence Electrons
Hydrogen has three isotopes: protium (¹H), deuterium (²H), and tritium (³H). These isotopes differ in the number of neutrons in their nuclei. However, all three isotopes possess one proton and one electron, maintaining their single valence electron. The difference in neutron numbers affects their mass and nuclear properties, but not their valence electron count or their fundamental chemical behavior.
Conclusion: The Significance of Hydrogen's Single Valence Electron
Hydrogen's single valence electron is the key to understanding its chemical behavior and its pervasive role in the universe. Its ability to form both covalent and ionic bonds, participate in various chemical reactions, and serve as a fundamental building block of countless molecules, is all a direct consequence of this single electron. Understanding hydrogen's atomic structure, including its single valence electron, is crucial for comprehending a vast array of chemical and physical phenomena. From the simplest molecules to complex biological systems, hydrogen's influence is undeniable, solidifying its importance as the first and most abundant element on the periodic table. Its unique position and properties continue to drive research and innovation across diverse scientific fields.
Latest Posts
Latest Posts
-
Phylogenetic Trees Are Used To Summarize
May 09, 2025
-
What Is Mercurys State Of Matter At Room Temperature
May 09, 2025
-
What Does The Graduated Cylinder Measure
May 09, 2025
-
Which Level Of Classification Is The Most Specific
May 09, 2025
-
Log X 4 Solve For X
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electros In Hydrogen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.