How Many Valence Electrons In N
listenit
Mar 15, 2025 · 6 min read
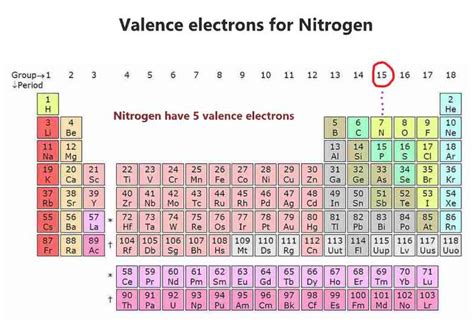
Table of Contents
How Many Valence Electrons Does Nitrogen (N) Have? A Deep Dive into Atomic Structure and Chemical Bonding
Nitrogen, a ubiquitous element crucial for life as we know it, plays a pivotal role in various biological and chemical processes. Understanding its electronic structure, particularly the number of valence electrons, is key to grasping its reactivity and the diverse range of compounds it forms. This article will delve deep into the question: How many valence electrons does nitrogen (N) have? We'll explore the underlying atomic theory, examine nitrogen's position on the periodic table, and discuss the implications of its valence electron configuration on its chemical behavior.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before we pinpoint the number of valence electrons in nitrogen, let's define the term. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound to the nucleus and are primarily responsible for an atom's chemical behavior. They participate in chemical bonding, determining how an atom interacts with other atoms to form molecules and compounds. The number of valence electrons significantly influences an atom's reactivity, its ability to gain, lose, or share electrons to achieve a stable electron configuration.
Nitrogen's Position on the Periodic Table: A Clue to its Valence Electrons
The periodic table is a powerful tool for predicting the properties of elements, including the number of valence electrons. Nitrogen (N) is located in Group 15 (also known as Group VA) of the periodic table. Group 15 elements are characterized by having five valence electrons. This is a crucial piece of information, giving us the immediate answer: Nitrogen has five valence electrons.
Delving into Nitrogen's Electron Configuration: A Closer Look
To understand why nitrogen has five valence electrons, let's examine its electron configuration. The electron configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. Nitrogen, with an atomic number of 7, has seven electrons in total. Its electron configuration is written as 1s²2s²2p³.
Let's break this down:
- 1s²: Two electrons occupy the first energy level (n=1) in the 's' subshell.
- 2s²: Two electrons occupy the second energy level (n=2) in the 's' subshell.
- 2p³: Three electrons occupy the second energy level (n=2) in the 'p' subshell.
The outermost shell of nitrogen is the second energy level (n=2). This shell contains a total of five electrons (two from the 2s subshell and three from the 2p subshell). These five electrons are the valence electrons of nitrogen.
The Significance of the Octet Rule: Stability Through Electron Sharing
The octet rule is a fundamental principle in chemistry that states atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight electrons in their outermost shell. This stable configuration resembles that of the noble gases, which are chemically inert.
Nitrogen, with only five valence electrons, is not stable. To achieve a stable octet, nitrogen typically forms covalent bonds by sharing its three unpaired electrons in the 2p subshell with other atoms. This sharing of electrons allows nitrogen to effectively "count" eight electrons in its valence shell, achieving a stable octet configuration.
Examples of Nitrogen's Chemical Bonding: Illustrating Valence Electron Participation
The five valence electrons of nitrogen dictate its diverse bonding capabilities. Let’s explore some examples:
1. Ammonia (NH₃): A Classic Example of Covalent Bonding
In ammonia (NH₃), nitrogen shares its three unpaired 2p electrons with three hydrogen atoms, each contributing one electron. This results in three covalent bonds, where each atom effectively achieves a stable octet configuration. Nitrogen shares three electrons and gains three electrons via sharing, resulting in a full octet. Each hydrogen atom gains one electron, filling its outermost shell (duet rule for hydrogen).
2. Nitrogen Gas (N₂): A Triple Bond for Exceptional Stability
Nitrogen gas (N₂), the most abundant component of Earth's atmosphere, showcases a remarkable example of covalent bonding. Two nitrogen atoms share three pairs of electrons, forming a strong triple bond (N≡N). This triple bond provides exceptional stability, explaining the inert nature of nitrogen gas under normal conditions. Each nitrogen atom contributes three electrons, creating a shared six electrons that fulfill the octet rule.
3. Nitric Oxide (NO): An Odd Electron Molecule
Nitric oxide (NO) is an example of a molecule with an odd number of electrons. Nitrogen contributes five valence electrons, and oxygen contributes six. This results in a total of eleven valence electrons, which means one electron remains unpaired. NO is thus a radical and relatively reactive compared to N₂ or NH₃.
4. Nitrate Ion (NO₃⁻): Resonance Structures and Delocalized Electrons
In the nitrate ion (NO₃⁻), nitrogen shares its five valence electrons with three oxygen atoms. The resulting structure involves resonance structures, where the double bond is delocalized among the three oxygen atoms. The formal charge is spread throughout the ion, contributing to its stability. Nitrogen achieves a full octet through the formation of covalent bonds, although the bonds are not localized in a single position.
Implications of Nitrogen's Valence Electrons: Beyond Chemical Bonding
The number of valence electrons in nitrogen isn't just crucial for understanding chemical bonding; it has profound implications across various fields:
-
Biological Systems: Nitrogen's ability to form diverse bonds is fundamental to the structure and function of biological molecules such as proteins, nucleic acids (DNA and RNA), and amino acids. The nitrogenous bases in DNA and RNA, for example, directly rely on nitrogen's valence electrons for their structure.
-
Industrial Applications: Nitrogen's reactivity is exploited in numerous industrial processes. Ammonia synthesis (Haber-Bosch process) utilizes nitrogen's capacity to form covalent bonds with hydrogen, producing a vital fertilizer. Nitrogen-containing compounds are also critical in the production of various materials, including explosives and pharmaceuticals.
-
Atmospheric Chemistry: Nitrogen's role in atmospheric chemistry is significant. Nitrogen gas constitutes the majority of the atmosphere, while nitrogen oxides play critical roles in air pollution and the formation of acid rain. Understanding nitrogen's reactivity is crucial for modeling and mitigating these atmospheric processes.
Conclusion: The Importance of Understanding Valence Electrons
Understanding the number of valence electrons in an atom is fundamental to comprehending its chemical behavior. Nitrogen, with its five valence electrons, exemplifies the importance of this concept. Its ability to form diverse covalent bonds, driven by its стремление to achieve a stable octet, explains its widespread presence in biological systems, industrial applications, and atmospheric processes. Its electron configuration and bonding characteristics are intertwined, shaping its unique and vital role in our world. From the air we breathe to the food we eat, nitrogen's five valence electrons are central to the chemistry of life and beyond.
Latest Posts
Latest Posts
-
8x 4 4x 3 4 6x 4 4
Mar 15, 2025
-
What Do The Arrows On A Food Chain Represent
Mar 15, 2025
-
Is Salt An Element Compound Or Mixture
Mar 15, 2025
-
How Many Millimeters Are In One Meter
Mar 15, 2025
-
What Is 1 2 3 8
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In N . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
