How Many Valence Electrons Does Cr Have
listenit
Mar 21, 2025 · 5 min read
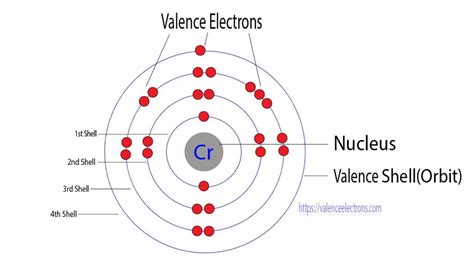
Table of Contents
How Many Valence Electrons Does Chromium (Cr) Have? Understanding Electronic Configuration and Chemical Behavior
Chromium (Cr), a lustrous, hard, and brittle transition metal, finds widespread applications ranging from stainless steel alloys to pigments and catalysts. Understanding its chemical behavior hinges on knowing its electronic configuration, particularly the number of valence electrons it possesses. This article delves deep into the intricacies of chromium's electron arrangement, explaining why determining its valence electrons isn't as straightforward as it might seem for other elements, and exploring the implications of this unique electronic structure.
Understanding Valence Electrons
Before we dive into the specifics of chromium, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are crucial because they are the ones involved in chemical bonding. They determine an element's reactivity and the types of chemical bonds it can form – be it ionic, covalent, or metallic. The number of valence electrons typically dictates the oxidation state an element can achieve.
For many elements, determining the number of valence electrons is straightforward. It's often equal to the group number (using the traditional numbering system) in the periodic table. However, transition metals like chromium present a more complex picture due to their unique electronic configurations.
Chromium's Electronic Configuration: The Anomaly
Chromium's atomic number is 24, indicating it possesses 24 electrons. One might expect its electronic configuration to be 1s²2s²2p⁶3s²3p⁶4s²3d⁴. However, this isn't the case. The actual electronic configuration of chromium is 1s²2s²2p⁶3s²3p⁶4s¹3d⁵. This deviation from the expected configuration is due to the exceptional stability associated with a half-filled d subshell.
A half-filled or fully-filled d subshell offers increased stability due to several factors:
-
Exchange Energy: Electrons with parallel spins (same spin quantum number) experience a repulsive force, but this repulsion is partly offset by exchange energy, a quantum mechanical effect that lowers the overall energy of the system. A half-filled d subshell maximizes this exchange energy.
-
Symmetrical Electron Distribution: A half-filled d subshell leads to a more symmetrical distribution of electrons, contributing to enhanced stability.
This extra stability is why one electron from the 4s orbital moves to the 3d orbital, resulting in a more stable configuration than the seemingly more logical 4s²3d⁴ arrangement.
So, How Many Valence Electrons Does Chromium Have?
This is where things get interesting. The definition of valence electrons becomes somewhat ambiguous when dealing with transition metals. While the outermost shell is the 4th shell in chromium, the 3d electrons are energetically close enough to participate in bonding.
Therefore, depending on the context and the specific chemical reaction, chromium can exhibit variable valency, meaning it can have different numbers of valence electrons participating in bonding. Considering both the 4s and 3d electrons, chromium can have up to six valence electrons.
However, it is more common to consider only the outermost s electrons as the valence electrons, in which case chromium would have only one valence electron (the 4s electron). The d electrons are often considered as inner-shell or core electrons despite the fact that they are involved in bonding. This is particularly true when chromium is in its lower oxidation states.
Chromium's Variable Oxidation States
The variable number of valence electrons directly relates to chromium's ability to exhibit a variety of oxidation states. Common oxidation states for chromium include:
-
+2 (Chromous): In this state, only the 4s electron is involved in bonding. This is a relatively uncommon oxidation state, as it is less stable than other options.
-
+3 (Chromic): This is a very common and stable oxidation state. It involves participation of the 4s electron and two of the 3d electrons.
-
+6 (Chromate/Dichromate): This is another significant oxidation state, commonly seen in compounds like chromate (CrO₄²⁻) and dichromate (Cr₂O₇²⁻). It involves the participation of all six valence electrons.
The oxidation state of chromium in a specific compound heavily depends on the electronegativity of the other atoms bonded to it.
Implications of Chromium's Electronic Configuration and Valence Electrons
The peculiar electronic configuration and the resulting variable valency of chromium have significant implications for its chemical properties and applications:
-
Catalysis: Chromium's ability to adopt multiple oxidation states makes it a versatile catalyst in various industrial processes, including polymerization and oxidation reactions. The change in oxidation state allows chromium to facilitate electron transfer between reactants.
-
Alloying: Chromium is a crucial alloying element in stainless steel, contributing significantly to its corrosion resistance. The variable valency influences the interaction between chromium and other metals in the alloy, creating a stable and protective oxide layer.
-
Pigments: Chromium compounds are used extensively as pigments due to their vibrant colors. For example, chromium(III) oxide (Cr₂O₃) is a green pigment, while lead chromate (PbCrO₄) is yellow. The color of these pigments is directly related to the electronic transitions within the chromium ion.
-
Biological Roles: While chromium is an essential trace element for human health, playing a role in glucose metabolism, its precise mechanisms and the relevant oxidation states are still under investigation.
Conclusion: A nuanced answer
To summarize, there isn't one single definitive answer to the question "How many valence electrons does chromium have?". While it has one electron in its outermost shell (4s), the nearby 3d electrons are also readily available for bonding, leading to variable valency. This unique feature allows chromium to participate in a wide variety of chemical reactions and contribute to its diverse applications in various fields. Understanding this nuance is crucial for comprehending chromium's fascinating and significant role in chemistry and materials science. Its variable valence electrons are the key to its exceptional versatility and make it a truly remarkable element. Further research into the intricacies of its electronic behavior continues to expand our understanding of this essential transition metal and its potential applications.
Latest Posts
Latest Posts
-
How Many Cups Is A Half A Gallon
Mar 28, 2025
-
The Plasma Membrane Of A Muscle Cell Is Called The
Mar 28, 2025
-
180 Inches Is How Many Yards
Mar 28, 2025
-
1 2x 1 2x X 1
Mar 28, 2025
-
What Is 7 20 As A Percent
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Cr Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
