How Many Valence Electrons Does Chloride Have
listenit
Mar 25, 2025 · 5 min read
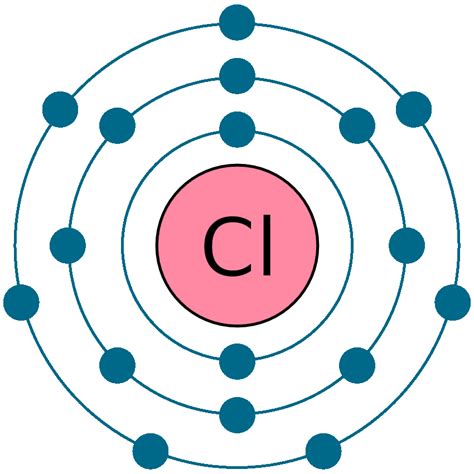
Table of Contents
How Many Valence Electrons Does Chloride Have? A Deep Dive into Chlorine's Reactivity
Understanding the number of valence electrons in an atom is fundamental to comprehending its chemical behavior. This is especially true for chlorine and its anion, chloride. This article will explore the concept of valence electrons, delve into the electronic structure of chlorine and chloride, and explain why knowing the number of valence electrons is crucial in predicting chemical reactions. We'll also discuss the implications of chloride's valence electrons in various contexts, from its role in biological systems to its industrial applications.
Understanding Valence Electrons
Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the ones involved in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons directly influences an atom's ability to gain, lose, or share electrons to achieve a stable electron configuration, typically resembling a noble gas. This stable configuration, often referred to as a full octet (eight electrons in the outermost shell), is the driving force behind many chemical reactions.
The Electronic Structure of Chlorine
Chlorine (Cl), with an atomic number of 17, possesses 17 electrons. These electrons are distributed across three energy levels:
- First energy level (n=1): 2 electrons
- Second energy level (n=2): 8 electrons
- Third energy level (n=3): 7 electrons
It's the electrons in the outermost energy level (n=3) that are the valence electrons. Therefore, chlorine has 7 valence electrons. This incomplete outermost shell makes chlorine highly reactive. It readily gains one electron to achieve a stable octet, resembling the noble gas Argon (Ar).
Visualizing Chlorine's Electron Configuration
A helpful way to visualize chlorine's electron configuration is using the electron shell diagram or the orbital notation. The electron shell diagram shows the electrons arranged in concentric circles around the nucleus. The orbital notation uses the principal quantum number (n), the azimuthal quantum number (l), and the magnetic quantum number (ml) to specify the location of the electrons.
The electronic configuration of chlorine is typically represented as: 1s²2s²2p⁶3s²3p⁵. This notation indicates that the first energy level has 2 electrons (1s²), the second level has 8 electrons (2s²2p⁶), and the third level has 7 electrons (3s²3p⁵). The 3s² and 3p⁵ subshells together contain the 7 valence electrons.
Chloride Ion (Cl⁻): Gaining Stability
When chlorine gains an electron, it forms a chloride ion (Cl⁻). This process is called reduction. The gained electron fills the 3p subshell, completing the octet in the outermost shell. The chloride ion therefore has 8 valence electrons. This stable configuration makes the chloride ion significantly less reactive than its neutral chlorine atom counterpart.
The Importance of the Octet Rule
The octet rule, a cornerstone of chemical bonding, states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight electrons in their outermost shell. Chlorine's eagerness to gain an electron to form chloride is a perfect example of this rule in action. This drive for stability is what dictates much of chlorine's chemical reactivity.
The Role of Valence Electrons in Chloride's Chemistry
The presence of eight valence electrons in the chloride ion fundamentally affects its chemical behavior. Since it has a complete octet, chloride ions are relatively unreactive and tend not to participate in further electron sharing or donation. This stability has significant consequences for the compounds that include chloride ions.
Ionic Bonding
Chloride's stability through achieving a full octet is particularly important in the formation of ionic bonds. Ionic bonds are formed when one atom transfers one or more electrons to another atom. The electrostatic attraction between the positively charged cation and the negatively charged anion (in this case, chloride) holds the compound together. Table salt (sodium chloride, NaCl) is a classic example of a compound formed via ionic bonding. Sodium (Na) readily loses one electron to achieve a stable octet, forming Na⁺, while chlorine gains this electron to become Cl⁻.
Covalent Bonding (Less Common)
While less common than ionic bonding, chloride can participate in covalent bonding under specific circumstances. Covalent bonds are formed when atoms share electrons to achieve a stable octet. These bonds are weaker than ionic bonds. However, the participation of chloride in covalent bonding is much less prominent than its role in ionic bonding due to its inherent stability.
Chloride's Importance in Biological Systems and Industrial Applications
The properties of the chloride ion, stemming directly from its eight valence electrons and resulting stability, make it crucial in many biological processes and industrial applications:
Biological Significance:
- Electrolyte Balance: Chloride ions are a major component of bodily fluids and play a crucial role in maintaining electrolyte balance. This balance is essential for proper nerve and muscle function.
- Digestion: Hydrochloric acid (HCl), found in the stomach, is vital for digestion, and chloride ions are a key component of this acid.
- Osmosis and Cell Volume: Chloride ions contribute to osmotic pressure, helping regulate the movement of water into and out of cells, thereby maintaining proper cell volume.
Industrial Applications:
- Production of PVC: Polyvinyl chloride (PVC), a widely used plastic, is made using chloride as a starting material.
- Water Purification: Chloride is used in some water purification processes to disinfect and remove impurities.
- Manufacturing of other Chemicals: Chloride is an essential component in the synthesis of numerous other chemicals used in various industries, including pharmaceuticals and agriculture.
Conclusion: Valence Electrons and the Reactivity of Chloride
The number of valence electrons is paramount in determining an atom's chemical reactivity. Chlorine, with its seven valence electrons, is highly reactive, readily gaining an electron to form the chloride ion (Cl⁻). This chloride ion, possessing eight valence electrons, achieves a stable octet, significantly reducing its reactivity. Understanding this fundamental difference between chlorine and chloride allows us to predict their behavior in various chemical reactions and appreciate their significance in diverse contexts, ranging from biological systems to industrial applications. The stability achieved by chloride through its full octet is the cornerstone of its diverse and vital roles in nature and industry. Knowing the number of valence electrons is therefore not merely an academic exercise; it’s a key to understanding the fundamental principles of chemistry and the world around us.
Latest Posts
Latest Posts
-
Through Which Medium Will Sound Travel Most Rapidly
Mar 25, 2025
-
Whats The Square Root Of 96
Mar 25, 2025
-
How Are The Electrons Arranged In An Atom
Mar 25, 2025
-
Is Sugar A Compound Or A Mixture
Mar 25, 2025
-
What Is The Reactant In Photosynthesis
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Chloride Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
