How Are The Electrons Arranged In An Atom
listenit
Mar 25, 2025 · 6 min read
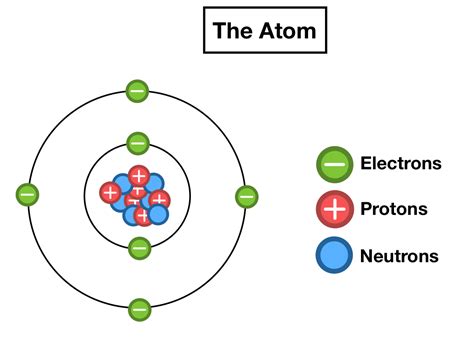
Table of Contents
How Are Electrons Arranged in an Atom? Unveiling the Secrets of Atomic Structure
Understanding the arrangement of electrons within an atom is fundamental to comprehending chemistry and the properties of matter. This seemingly simple question leads us down a fascinating path exploring quantum mechanics, energy levels, orbitals, and the periodic table itself. This article will delve deep into the intricacies of electron arrangement, explaining the underlying principles and their implications.
The Bohr Model: A Simplified Introduction
While outdated in its details, the Bohr model provides a useful starting point for visualizing electron arrangement. This model depicts electrons orbiting the nucleus in distinct energy levels or shells. The closer a shell is to the nucleus, the lower its energy. Each shell can hold a specific number of electrons:
- Shell 1 (K shell): Holds a maximum of 2 electrons
- Shell 2 (L shell): Holds a maximum of 8 electrons
- Shell 3 (M shell): Holds a maximum of 18 electrons
- Shell 4 (N shell): Holds a maximum of 32 electrons
and so on. The electrons fill these shells sequentially, starting with the lowest energy level. For example, Lithium (Li), with three electrons, has two electrons in the K shell and one in the L shell.
Limitations of the Bohr Model: The Bohr model, while helpful for basic understanding, significantly oversimplifies the reality of electron behavior. It fails to explain the spectra of atoms with more than one electron and doesn't account for the wave-particle duality of electrons.
The Quantum Mechanical Model: A More Accurate Picture
The quantum mechanical model provides a much more accurate and comprehensive description of electron arrangement. It relies on the principles of quantum mechanics, which state that electrons don't orbit the nucleus in neat, defined paths like planets around a sun. Instead, they exist in atomic orbitals, regions of space where there is a high probability of finding an electron.
Quantum Numbers: Defining an Electron's State
To fully describe the location and energy of an electron within an atom, we use four quantum numbers:
-
Principal Quantum Number (n): This number determines the electron's energy level and the size of the orbital. It's a positive integer (n = 1, 2, 3,...), corresponding to the shells in the Bohr model (n=1 is the K shell, n=2 is the L shell, and so on). Higher values of 'n' mean higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This number specifies the shape of the orbital and its angular momentum. It can have integer values from 0 to n-1. For example, if n=2, l can be 0 or 1. These values correspond to different subshells:
- l = 0: s orbital (spherical shape)
- l = 1: p orbital (dumbbell shape)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
-
Magnetic Quantum Number (ml): This number describes the orientation of the orbital in space. It can have integer values from -l to +l, including 0. For example, if l=1 (p orbital), ml can be -1, 0, or +1, representing three p orbitals oriented along the x, y, and z axes, respectively.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, often referred to as its "spin." It can have only two values: +1/2 (spin up) or -1/2 (spin down). This is crucial for understanding electron pairing within orbitals.
Electron Configuration: Filling the Orbitals
Electron configuration describes how electrons are distributed among the various orbitals within an atom. It follows specific rules:
-
Aufbau Principle: Electrons fill orbitals starting with the lowest energy level and moving to higher levels.
-
Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, and these electrons must have opposite spins.
-
Hund's Rule: When filling orbitals of equal energy (degenerate orbitals, like the three p orbitals), electrons first occupy each orbital singly with parallel spins before pairing up.
Let's consider the electron configuration of Nitrogen (N), which has seven electrons:
1s² 2s² 2p³
This notation means:
- Two electrons in the 1s orbital (n=1, l=0, ml=0)
- Two electrons in the 2s orbital (n=2, l=0, ml=0)
- Three electrons in the 2p orbitals (n=2, l=1, ml = -1, 0, +1). According to Hund's rule, these three electrons will occupy each of the three 2p orbitals individually before pairing up.
The Periodic Table and Electron Configuration
The periodic table is organized based on the electron configurations of elements. Elements in the same group (vertical column) have similar electron configurations in their outermost shell (valence shell), leading to similar chemical properties.
- Group 1 (Alkali Metals): All have one electron in their valence s orbital (ns¹).
- Group 18 (Noble Gases): All have a completely filled valence shell, resulting in exceptional stability.
The periodic table's structure reflects the filling order of orbitals, with blocks corresponding to specific subshells:
- s-block: Groups 1 and 2 (alkali and alkaline earth metals)
- p-block: Groups 13-18
- d-block: Transition metals
- f-block: Lanthanides and actinides
Exceptions to the Rules
While the Aufbau principle, Pauli exclusion principle, and Hund's rule generally predict electron configurations accurately, some exceptions exist. These exceptions often arise due to the close energy levels of certain orbitals, leading to subtle energy shifts that favor different electron arrangements. For instance, Chromium (Cr) and Copper (Cu) have unusual electron configurations that deviate from the expected pattern due to the enhanced stability associated with half-filled and completely filled subshells.
Beyond Basic Electron Configuration
The concepts discussed so far provide a fundamental understanding of electron arrangement. However, a deeper dive into atomic structure reveals further complexities:
- Electron correlation: The mutual interaction between electrons, influencing their distribution and energy levels.
- Electron shielding: The effect of inner electrons on the attraction between outer electrons and the nucleus.
- Effective nuclear charge: The net positive charge experienced by an outer electron, considering the shielding effect.
These factors play a crucial role in determining the chemical behavior of elements and their interactions with other atoms.
Applications and Significance
Understanding electron arrangement is crucial for various scientific disciplines:
- Chemistry: Predicting chemical bonding, reactivity, and the properties of molecules.
- Materials Science: Designing new materials with specific properties by manipulating electron configurations.
- Spectroscopy: Analyzing the interaction of light with matter based on electron transitions between energy levels.
- Nuclear Physics: Understanding nuclear reactions and radioactive decay.
Conclusion
The arrangement of electrons in an atom is a complex yet fascinating topic. While the Bohr model offers a simplified visualization, the quantum mechanical model provides a more accurate and complete picture. The four quantum numbers, coupled with the Aufbau principle, Pauli exclusion principle, and Hund's rule, enable us to predict and understand the electron configurations of various elements and their resulting properties. This knowledge is fundamental to various scientific fields and serves as a cornerstone for understanding the behavior of matter at the atomic level. Further exploration of advanced concepts like electron correlation and effective nuclear charge deepens our understanding of this intricate aspect of atomic structure.
Latest Posts
Latest Posts
-
Least Common Multiple Of 35 And 25
Mar 27, 2025
-
When A Sperm And Egg Combine It Is Called
Mar 27, 2025
-
What Is The Square Root Of 484
Mar 27, 2025
-
What Is 2 To The Negative 3rd Power
Mar 27, 2025
-
Square Root Of 125 In Radical Form
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How Are The Electrons Arranged In An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
