How Many Valence Electrons Does Ba Have
listenit
Mar 20, 2025 · 5 min read
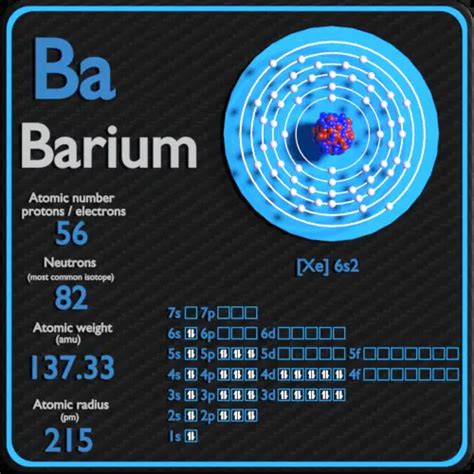
Table of Contents
How Many Valence Electrons Does Ba Have? Understanding Barium's Reactivity
Barium (Ba), a shiny, silvery-white alkaline earth metal, plays a fascinating role in chemistry, largely due to its electronic structure. Understanding its valence electrons is key to comprehending its reactivity and the types of chemical bonds it forms. So, how many valence electrons does barium have? The answer, as we'll explore in detail, is two. But let's delve deeper to understand why this number is so crucial and what it means for barium's behavior.
Understanding Valence Electrons
Before we pinpoint the number of valence electrons in barium, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely held and are therefore the ones involved in chemical bonding. They determine an element's reactivity, its ability to form chemical bonds with other atoms, and its overall chemical properties. Essentially, they dictate how an atom interacts with its environment at a chemical level.
Barium's Electronic Configuration: The Key to Valence Electrons
To determine the number of valence electrons in barium, we need to examine its electronic configuration. The electronic configuration describes how electrons are distributed among the different energy levels (shells) and sublevels (orbitals) within an atom. Barium's atomic number is 56, meaning it has 56 protons and 56 electrons in a neutral atom. Its electronic configuration is:
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²
This configuration indicates the arrangement of electrons in different energy levels. The superscripts represent the number of electrons in each subshell.
Identifying Valence Electrons in Barium's Configuration
The outermost shell of an atom is the one with the highest principal quantum number (n). In barium's configuration, the highest principal quantum number is 6. The electrons in this shell (n=6) are the valence electrons. Looking at the configuration, we see that the 6s subshell contains two electrons (6s²).
Therefore, barium has two valence electrons.
The Significance of Two Valence Electrons
The presence of two valence electrons is crucial in understanding barium's chemical behavior. Alkaline earth metals, like barium, are characterized by their two valence electrons. This configuration makes them highly reactive, as they readily lose these two electrons to achieve a stable octet (eight electrons) in their outermost shell, mimicking the electron configuration of a noble gas. This process of losing electrons is known as oxidation.
Chemical Reactions and Barium's Valence Electrons
The two valence electrons in barium dictate its participation in chemical reactions:
-
Ionic Bonding: Barium readily loses its two valence electrons to form a +2 ion (Ba²⁺). This ion then forms ionic bonds with negatively charged ions, such as chloride (Cl⁻) to form barium chloride (BaCl₂). The electrostatic attraction between the positively charged barium ion and the negatively charged chloride ions holds the compound together.
-
Metallic Bonding: Barium exhibits metallic bonding in its pure elemental state. The valence electrons are delocalized, forming a "sea" of electrons that are shared among the barium atoms. This shared electron sea allows for electrical and thermal conductivity, characteristic of metals.
-
Reactivity with Water and Acids: Barium reacts vigorously with water and acids, due to its eagerness to lose its valence electrons. The reaction with water produces barium hydroxide and hydrogen gas. The reaction with acids produces barium salts and hydrogen gas. These reactions are highly exothermic, meaning they release significant heat.
-
Limited Covalent Bonding: While primarily known for ionic bonding, barium can participate in some covalent bonding, although this is less common than ionic bonding.
Applications Leveraging Barium's Reactivity
The unique properties of barium, stemming from its two valence electrons and subsequent reactivity, lead to its use in various applications:
-
Medical Imaging: Barium sulfate (BaSO₄) is used as a contrast agent in medical imaging, particularly in X-ray examinations of the digestive system. Its high atomic number and insolubility in water make it ideal for this purpose.
-
Pyrotechnics: Barium compounds are used in pyrotechnics to produce a bright green color in fireworks. The excitation of barium electrons during combustion emits light at specific wavelengths, resulting in the characteristic green glow.
-
Vacuum Tubes: Barium's ability to absorb gases is utilized in vacuum tubes. It acts as a getter, removing residual gases from the tube to improve its performance.
-
Alloys: Barium is added to certain alloys to enhance their properties, such as improving their machinability and ductility.
Safety Considerations with Barium
It's crucial to acknowledge the safety aspects related to handling barium and its compounds. Barium is a reactive metal and some of its compounds are toxic. Appropriate safety precautions, including protective equipment, must be taken when working with barium to minimize health risks. Appropriate handling and disposal procedures are essential.
Conclusion: The Importance of Valence Electrons in Understanding Barium
The presence of two valence electrons fundamentally defines barium's chemical behavior. This characteristic is responsible for its reactivity, its ability to form ionic bonds, its role in various industrial applications, and its unique properties that make it valuable in different fields. Understanding the electronic configuration and the significance of valence electrons is essential for predicting and comprehending the chemical and physical behavior of barium and other elements. This knowledge is not just theoretical; it is directly applicable in understanding the real-world uses and implications of this fascinating element. Remember always to prioritize safety when working with barium or any chemically reactive substance.
Latest Posts
Latest Posts
-
X 3 3x 2 4x 12
Mar 21, 2025
-
What Is The Lowest Common Multiple Of 15 And 20
Mar 21, 2025
-
Can Crawfish Live Out Of Water
Mar 21, 2025
-
Whats The Difference Between Static And Current Electricity
Mar 21, 2025
-
Least Common Multiple Of 3 8
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Ba Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
