How Many Valence Electrons Are In Iron
listenit
Mar 18, 2025 · 6 min read
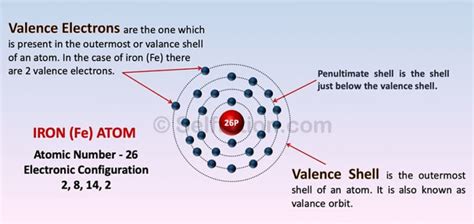
Table of Contents
How Many Valence Electrons Are in Iron? Understanding Electronic Configuration and Chemical Behavior
Iron, a ubiquitous element crucial to life and industry, presents an intriguing case study in understanding electron configuration and valence electrons. While seemingly straightforward, accurately determining the number of valence electrons in iron requires delving into the complexities of its electronic structure and considering its diverse chemical behavior. This article will comprehensively explore the answer to the question: how many valence electrons are in iron? We'll investigate its electronic configuration, oxidation states, and the implications of its valence electrons on its reactivity and applications.
Understanding Valence Electrons
Before diving into iron's specifics, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial in determining an element's chemical properties, as they are involved in the formation of chemical bonds with other atoms. The number of valence electrons dictates an element's reactivity, the types of bonds it can form (ionic, covalent, metallic), and its overall chemical behavior.
Iron's Electronic Configuration: The Key to Understanding Valence Electrons
To ascertain the number of valence electrons in iron (Fe), we must examine its electronic configuration. Iron, with an atomic number of 26, possesses 26 electrons. These electrons fill the atomic orbitals according to the Aufbau principle and Hund's rule, resulting in the following electronic configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶
This configuration reveals that iron's electrons occupy various energy levels (shells) and sub-shells. The principal quantum number (n) designates the energy level, while the azimuthal quantum number (l) determines the subshell (s, p, d, f).
Analyzing the electronic configuration, we observe that the outermost shell is the fourth shell (n=4). This shell contains two electrons in the 4s subshell. However, the story doesn't end there. The 3d subshell, while technically an inner shell, also plays a significant role in iron's chemical behavior. This is because the energy difference between the 4s and 3d orbitals is relatively small, allowing 3d electrons to participate in chemical bonding.
The Variable Valence of Iron: Oxidation States
Unlike elements with clearly defined valence electrons, iron exhibits variable valence. This means that it can lose a different number of electrons depending on the chemical environment. This phenomenon arises because the energy levels of the 3d and 4s electrons are close, making it energetically feasible for iron to lose varying numbers of electrons to achieve stability.
The most common oxidation states of iron are +2 (ferrous) and +3 (ferric). Let's examine each:
Iron(II) (Ferrous): Losing Two Electrons
In the +2 oxidation state, iron loses two electrons, typically the two 4s electrons. The electronic configuration becomes: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶. In this state, it effectively has zero valence electrons from the perspective of the outermost shell.
Iron(III) (Ferric): Losing Three Electrons
In the +3 oxidation state, iron loses three electrons. This involves losing the two 4s electrons and one 3d electron. The electronic configuration becomes: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁵. Similar to Iron(II), in this state, it effectively has zero valence electrons from the perspective of the outermost shell.
Higher Oxidation States: Rarity but Significance
While less common, iron can also exhibit higher oxidation states, such as +4, +5, and +6. In these instances, the involvement of 3d and potentially even 3p electrons becomes crucial in forming chemical bonds. The exact number of valence electrons in these higher oxidation states depends on the specific chemical context and the nature of the bond formation.
Implications of Iron's Valence Electrons
The variable valence of iron profoundly influences its chemical behavior and diverse applications.
Chemical Reactivity: A Balancing Act
The ability of iron to lose different numbers of electrons makes it highly reactive. This reactivity is central to its role in various chemical processes, including:
-
Oxidation and Reduction Reactions: Iron readily participates in redox reactions, switching between its +2 and +3 oxidation states, acting as an oxidizing or reducing agent depending on the circumstances. This is fundamental to its use in batteries and other electrochemical applications.
-
Complex Formation: Iron's ability to form coordination complexes with ligands is extensively exploited in chemistry and biochemistry. The 3d orbitals play a crucial role in forming these complexes, influencing their properties and applications.
-
Catalysis: Iron's variable oxidation states make it a vital component of numerous catalysts, facilitating a wide range of chemical reactions in industry and biological systems. Examples include the Haber-Bosch process for ammonia synthesis and various enzyme-catalyzed reactions.
Biological Significance: Life's Essential Metal
Iron's chemical properties are inextricably linked to its crucial role in biological systems. It's a central component of:
-
Hemoglobin: This protein, responsible for oxygen transport in blood, utilizes iron in its heme group to bind and release oxygen molecules. The iron's ability to switch between oxidation states is critical for this process.
-
Myoglobin: This protein stores oxygen in muscle tissue, similarly utilizing iron in a heme group.
-
Cytochromes: These proteins participate in electron transport chains in cellular respiration, using iron to facilitate electron transfer.
Industrial Applications: From Steel to Catalysts
Iron's unique properties have resulted in its widespread use in various industrial applications:
-
Steel Production: Iron is the primary component of steel, an alloy renowned for its strength and versatility. The addition of other elements modifies steel's properties, leading to a vast array of applications.
-
Pigments: Iron oxides are used extensively as pigments in paints, cosmetics, and other materials, contributing diverse colors and properties.
-
Catalysis: Iron-based catalysts are used in numerous industrial processes, including ammonia synthesis, petroleum refining, and the production of various chemicals.
Conclusion: Beyond a Simple Number
Determining the number of valence electrons in iron isn't a simple case of counting electrons in the outermost shell. The proximity in energy levels of the 3d and 4s electrons, coupled with the element's ability to achieve multiple oxidation states, complicates the picture. While in the +2 and +3 oxidation states, it might seem to have zero valence electrons in the outermost shell, the participation of the 3d electrons in chemical bonding indicates a more nuanced understanding is required. Ultimately, iron's variable valence and the involvement of both 4s and 3d electrons are responsible for its rich chemistry, crucial biological functions, and wide range of industrial applications. Understanding its electronic configuration and the implications of this variable valence is crucial to comprehending iron's pivotal role in both the natural world and human technology.
Latest Posts
Latest Posts
-
Meters Per Minute To Miles Per Hour
Mar 18, 2025
-
How Many Quarts Is 48 Ounces
Mar 18, 2025
-
Write The Polynomial In Standard Form
Mar 18, 2025
-
The Stomach Is Inferior To The Diaphragm
Mar 18, 2025
-
Is Rotting A Physical Or Chemical Change
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Iron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
