How Many Total Atoms Are In 4ca3 Po4 2
listenit
Mar 16, 2025 · 4 min read
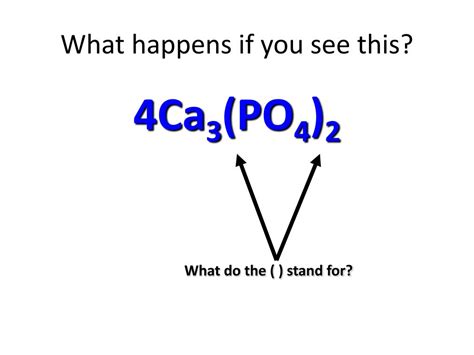
Table of Contents
How Many Total Atoms Are in 4Ca₃(PO₄)₂? A Deep Dive into Chemical Calculations
Determining the total number of atoms in a given chemical formula requires a thorough understanding of stoichiometry. This article will guide you through the process of calculating the total number of atoms in four units of calcium phosphate, Ca₃(PO₄)₂, providing a detailed explanation of each step and incorporating relevant chemical concepts. We'll explore not only the calculation itself but also the broader context of chemical formulas and Avogadro's number.
Understanding the Chemical Formula: Ca₃(PO₄)₂
Before embarking on the calculation, let's dissect the chemical formula Ca₃(PO₄)₂. This represents calcium phosphate, a common mineral found in bones and teeth. Let's break down its components:
-
Ca: This represents the element calcium. The subscript '3' indicates that there are three calcium atoms in one formula unit of calcium phosphate.
-
(PO₄): This is a phosphate ion, a polyatomic ion composed of one phosphorus atom (P) and four oxygen atoms (O). The parentheses indicate that this entire group is treated as a single unit.
-
₂: The subscript '2' outside the parentheses means that there are two phosphate ions in one formula unit of calcium phosphate.
Step-by-Step Calculation: Atoms in One Formula Unit
First, we'll calculate the total number of atoms in a single formula unit of Ca₃(PO₄)₂:
-
Calcium Atoms: There are 3 calcium atoms per formula unit.
-
Phosphorus Atoms: Each phosphate ion (PO₄) contains one phosphorus atom, and there are two phosphate ions, resulting in 2 phosphorus atoms per formula unit.
-
Oxygen Atoms: Each phosphate ion (PO₄) contains four oxygen atoms. With two phosphate ions, there are a total of 2 * 4 = 8 oxygen atoms per formula unit.
-
Total Atoms per Formula Unit: Adding the number of atoms of each element: 3 (Ca) + 2 (P) + 8 (O) = 13 atoms per formula unit.
Calculating Atoms in Four Formula Units of Ca₃(PO₄)₂
Now, we'll extend our calculation to determine the total number of atoms in four formula units of calcium phosphate:
Since there are 13 atoms in one formula unit, and we have four formula units, the total number of atoms is:
13 atoms/formula unit * 4 formula units = 52 atoms
Therefore, there are a total of 52 atoms in 4Ca₃(PO₄)₂.
Beyond the Basic Calculation: Introducing Moles and Avogadro's Number
While the above calculation provides the total number of atoms in four formula units, it doesn't represent a macroscopic quantity. In chemistry, we often deal with vast numbers of atoms and molecules, which is where the concept of moles and Avogadro's number comes into play.
Avogadro's number (approximately 6.022 x 10²³) is the number of entities (atoms, molecules, ions, etc.) in one mole of a substance. A mole is a unit of measurement in chemistry that relates the mass of a substance to the number of particles it contains.
To calculate the total number of atoms in four moles of Ca₃(PO₄)₂, for instance, we would first determine the number of formula units in four moles:
4 moles * 6.022 x 10²³ formula units/mole = 2.4088 x 10²⁴ formula units
Then, we would multiply this by the number of atoms per formula unit (13):
2.4088 x 10²⁴ formula units * 13 atoms/formula unit ≈ 3.13 x 10²⁵ atoms
This demonstrates the sheer scale of the numbers involved when dealing with molar quantities of chemical compounds.
Practical Applications and Further Exploration
The ability to calculate the number of atoms in a given amount of a substance is crucial in various fields, including:
-
Chemistry: Stoichiometric calculations are fundamental to understanding chemical reactions and determining the amounts of reactants and products.
-
Materials Science: Calculating the number of atoms is vital for understanding the properties and behavior of materials at the atomic level.
-
Biochemistry: In biochemistry, accurately calculating the number of atoms is essential for understanding the structure and function of biological molecules.
-
Pharmaceutical Industry: Precise calculations are crucial for dosage control and drug formulation.
Expanding Your Knowledge: Related Concepts
To further enhance your understanding, exploring these related concepts is highly recommended:
-
Molar Mass: Understanding how to calculate the molar mass of a compound is crucial for converting between mass and moles.
-
Percent Composition: Learning to calculate the percent composition of a compound allows you to determine the relative amounts of each element present.
-
Empirical and Molecular Formulas: Understanding the differences between empirical and molecular formulas is important for interpreting chemical data.
-
Stoichiometry Problems: Practicing a variety of stoichiometry problems will solidify your understanding and problem-solving skills.
Conclusion: Mastering Atomic Calculations
Accurately determining the number of atoms in a chemical compound is a cornerstone of chemistry. This article has provided a step-by-step guide to calculating the total number of atoms in 4Ca₃(PO₄)₂, highlighting the importance of understanding chemical formulas, applying Avogadro's number, and appreciating the practical applications of these calculations in various scientific fields. By mastering these fundamental concepts, you can confidently tackle more complex stoichiometry problems and gain a deeper understanding of the world at the atomic level. Remember to practice regularly and explore further related concepts to build a solid foundation in chemical calculations.
Latest Posts
Latest Posts
-
Does Australia Use Fahrenheit Or Celsius
Mar 16, 2025
-
What Are The Rows On The Periodic Table Called
Mar 16, 2025
-
Words That Start With The Same Letter
Mar 16, 2025
-
What Is The Derivative Of 3 X
Mar 16, 2025
-
Least Common Multiple For 4 And 7
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about How Many Total Atoms Are In 4ca3 Po4 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
