How Many Protons Neutrons And Electrons Does Boron Have
listenit
Mar 30, 2025 · 5 min read
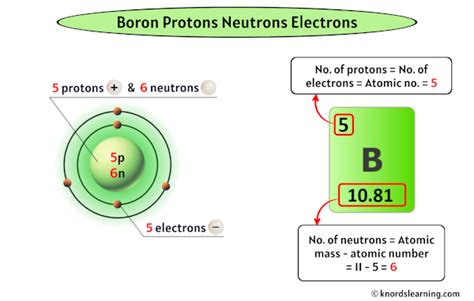
Table of Contents
How Many Protons, Neutrons, and Electrons Does Boron Have? A Deep Dive into Atomic Structure
Boron, a metalloid element with the symbol B and atomic number 5, holds a unique position in the periodic table. Understanding its atomic structure—specifically, the number of protons, neutrons, and electrons—is fundamental to comprehending its chemical properties and behavior. This article will delve deep into the atomic composition of boron, exploring its isotopes, their implications, and the broader context of atomic structure.
Understanding Atomic Structure: The Basics
Before diving into boron's specific composition, let's briefly review the fundamental components of an atom:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element; all boron atoms have 5 protons.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. In a neutral atom, the number of electrons equals the number of protons.
The mass number of an atom is the sum of its protons and neutrons. This is crucial because it distinguishes between isotopes of the same element.
Boron's Atomic Composition: Protons, Neutrons, and Electrons
As mentioned, boron always has 5 protons. This is non-negotiable; it's what makes it boron. The number of electrons in a neutral boron atom is also 5, balancing the positive charge of the protons.
However, the number of neutrons is where things get interesting. Boron exists in nature as a mixture of two stable isotopes:
- Boron-10 (¹⁰B): This isotope contains 5 protons and 5 neutrons. Its mass number is 10 (5 protons + 5 neutrons).
- Boron-11 (¹¹B): This isotope contains 5 protons and 6 neutrons. Its mass number is 11 (5 protons + 6 neutrons).
The abundance of these isotopes varies naturally. Boron-11 is significantly more prevalent, making up approximately 80% of naturally occurring boron. The remaining 20% is Boron-10. This isotopic variation influences the average atomic mass of boron, which is approximately 10.81 atomic mass units (amu). This average reflects the weighted contribution of both isotopes.
Visualizing Boron's Isotopes
Imagine two slightly different boron atoms:
- Atom 1 (¹⁰B): Nucleus with 5 protons and 5 neutrons, surrounded by 5 electrons.
- Atom 2 (¹¹B): Nucleus with 5 protons and 6 neutrons, surrounded by 5 electrons.
Both are boron atoms because they share the same number of protons. The difference lies solely in the number of neutrons, affecting the atom's mass but not its chemical properties significantly.
The Significance of Isotopes in Boron's Properties
The presence of two stable isotopes has consequences for boron's properties and applications:
- Average Atomic Mass: The weighted average of the masses of its isotopes determines boron's average atomic mass used in chemical calculations.
- Nuclear Applications: The different neutron numbers affect how each isotope interacts with neutrons. Boron-10, in particular, has a high neutron absorption cross-section, making it crucial in nuclear reactors as a neutron absorber in control rods. This helps regulate the rate of nuclear fission. This property is exploited to control the chain reaction and prevent accidents.
- Chemical Behavior: While the number of neutrons affects mass, it doesn't drastically change boron's chemical behavior. Both isotopes react similarly in chemical reactions because they have the same number of valence electrons (electrons in the outermost shell), which determine an element's reactivity. The slight mass difference may lead to some kinetic isotope effects, but these are generally minor.
- Isotopic Enrichment: Because Boron-10 has specific applications, methods exist to enrich boron samples, increasing the proportion of Boron-10 above its natural abundance. This enrichment process is energy-intensive and technologically demanding.
Boron's Electron Configuration and Chemical Bonding
Boron's electron configuration is 1s²2s²2p¹. This means that:
- The first energy level (n=1) contains two electrons in the 1s orbital.
- The second energy level (n=2) contains three electrons: two in the 2s orbital and one in the 2p orbital.
This single electron in the 2p orbital is crucial for boron's chemical behavior. Boron readily forms three covalent bonds, sharing this electron with other atoms to achieve a stable electron configuration. This is why boron commonly exhibits a +3 oxidation state.
Examples of Boron's Bonding
Boron forms diverse compounds due to its ability to form covalent bonds. Here are some examples:
- Boron trifluoride (BF₃): Boron shares its three valence electrons with three fluorine atoms, forming three covalent bonds.
- Borax (Na₂B₄O₇·10H₂O): A common boron compound found in nature, exhibiting complex bonding arrangements involving boron-oxygen bonds.
- Boron carbide (B₄C): A hard and extremely strong material with a complex bonding network.
These examples demonstrate boron's versatility in forming compounds with diverse properties, ranging from relatively simple molecules to complex solids.
Applications of Boron and its Isotopes
The unique properties of boron and its isotopes lead to a wide range of applications:
- Nuclear Reactors: Boron-10's high neutron absorption cross-section makes it crucial in controlling nuclear fission reactions.
- Doping in Semiconductors: Boron is used as a dopant in semiconductors, enhancing their conductivity.
- Glass and Ceramics: Boron compounds are essential components in various glasses and ceramics, improving their strength, durability, and other properties.
- Agriculture: Boron is an essential micronutrient for plants, crucial for healthy growth and development. Deficiencies can lead to severe yield reductions.
- Medicines: Boron compounds have applications in medicine, including boron neutron capture therapy (BNCT), a type of cancer treatment.
Conclusion: A Comprehensive Understanding of Boron
In summary, boron, with its atomic number 5, possesses 5 protons and 5 electrons in its neutral state. The presence of two stable isotopes, Boron-10 and Boron-11, each differing in neutron count, significantly impacts its average atomic mass and applications, especially in nuclear technology. Understanding boron's atomic structure, isotopic variation, and electron configuration is crucial for appreciating its diverse chemical behavior and wide-ranging applications across various industries, from nuclear energy to agriculture and medicine. The unique properties of this element continue to fuel scientific advancements and technological innovations.
Latest Posts
Latest Posts
-
Weight Of A Dime In Grams
Apr 01, 2025
-
How Many Yards Are In A Square Mile
Apr 01, 2025
-
What Is 1 16 As A Percentage
Apr 01, 2025
-
What Energy Conversion Occurs During Photosynthesis
Apr 01, 2025
-
How Many Valence Electrons Are In I
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Neutrons And Electrons Does Boron Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
