How Many Protons Are In Gold
listenit
Mar 16, 2025 · 5 min read
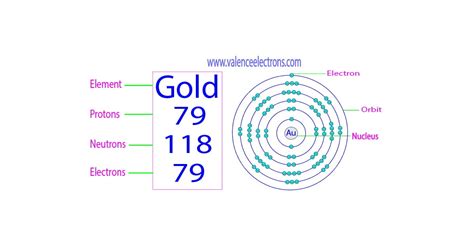
Table of Contents
How Many Protons are in Gold? Delving into Atomic Structure and Isotopes
Gold, a lustrous and highly valued metal, holds a fascinating place in human history and scientific understanding. But beyond its cultural significance and economic importance lies a fundamental question about its atomic structure: how many protons are in gold? This seemingly simple question opens the door to a deeper exploration of atomic theory, isotopes, and the periodic table.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we answer the central question, let's establish a firm grasp of atomic structure. An atom, the basic unit of a chemical element, is composed of three subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element; it's the atomic number.
- Neutrons: Neutral particles (no charge) also found in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. Their number typically equals the number of protons in a neutral atom.
The number of protons, neutrons, and electrons fundamentally dictates an atom's properties and behavior. The mass number of an atom is the sum of its protons and neutrons.
The Atomic Number of Gold: Unveiling the Proton Count
Gold, represented by the chemical symbol Au (from the Latin aurum), occupies a unique position on the periodic table. Its atomic number is 79. This crucial piece of information directly answers our initial question: there are 79 protons in a gold atom. This is a defining characteristic of gold; any atom with 79 protons is, by definition, a gold atom. No other element possesses this precise number of protons.
The Significance of the Atomic Number
The atomic number is not merely a numerical label; it's a fundamental property that dictates the element's chemical behavior. The number of protons determines:
- Chemical Properties: The arrangement of electrons, dictated by the number of protons, governs how an atom interacts with other atoms, forming chemical bonds. Gold's unique electron configuration accounts for its inertness and resistance to corrosion.
- Position on the Periodic Table: The periodic table is organized by atomic number, placing elements with similar properties in the same groups or columns. Gold's position reflects its characteristics as a transition metal.
- Isotope Identification: While the number of protons remains constant for a given element, the number of neutrons can vary, leading to isotopes.
Isotopes of Gold: Variations in Neutron Count
While the number of protons in gold is always 79, the number of neutrons can differ. These variations are called isotopes. Isotopes of the same element have the same number of protons but different numbers of neutrons. This results in different mass numbers but identical chemical properties.
Gold has several known isotopes, most of which are unstable and radioactive. The most common and stable isotope is Gold-197 (¹⁹⁷Au). This isotope accounts for nearly all naturally occurring gold.
- ¹⁹⁷Au: This isotope has 79 protons and 118 neutrons (79 + 118 = 197). It's stable and non-radioactive, making up the bulk of gold found in nature.
- Other Gold Isotopes: Other gold isotopes, such as ¹⁹⁵Au, ¹⁹⁸Au, and several others, are radioactive and decay over time. These isotopes have applications in various fields, including medicine and research.
Understanding Isotopic Abundance
The abundance of different isotopes in a naturally occurring sample of an element varies. For gold, ¹⁹⁷Au has an abundance of nearly 100%, meaning virtually all naturally occurring gold consists of this isotope. The presence of other, less abundant isotopes has minimal impact on gold's overall properties.
The Role of Protons in Gold's Properties
The presence of 79 protons in gold directly influences its properties, making it the unique metal we know and value:
- Malleability and Ductility: Gold's atomic structure allows its atoms to slide past each other easily, resulting in its exceptional malleability (ability to be hammered into thin sheets) and ductility (ability to be drawn into wires).
- Conductivity: The mobile electrons in gold's electron shells contribute to its excellent electrical and thermal conductivity.
- Inertness: Gold's resistance to oxidation and corrosion is partly attributed to its electron configuration and the strong metallic bonds between its atoms.
- Density: Gold's high density is a result of the large number of protons and neutrons in its nucleus, packing a significant amount of mass into a small volume.
- Color and Lustre: The specific arrangement of electrons and their interactions with light are responsible for gold's characteristic yellow color and metallic luster.
Gold in Science and Technology
Gold's unique properties have led to its extensive use in various scientific and technological applications:
- Electronics: Gold's conductivity and resistance to corrosion make it ideal for use in electrical contacts and connectors.
- Medicine: Gold compounds have been used in treating certain medical conditions, such as rheumatoid arthritis. Radioactive gold isotopes find applications in medical imaging and treatment.
- Dentistry: Gold alloys are used in dental fillings and crowns due to their biocompatibility and durability.
- Catalysis: Gold nanoparticles exhibit remarkable catalytic properties, finding applications in various chemical processes.
- Space Exploration: Gold's resistance to corrosion and high thermal conductivity makes it valuable in space exploration technology.
Conclusion: The Fundamental Importance of Protons
The seemingly simple question, "How many protons are in gold?", serves as a gateway to understanding the intricacies of atomic structure and the periodic table. The presence of 79 protons defines gold, dictating its unique properties and applications. Understanding isotopes further enriches our knowledge, revealing the variations within the gold element itself. From its cultural significance to its indispensable role in scientific advancements, gold's properties are fundamentally rooted in its atomic structure and the specific number of protons within its nucleus. This knowledge underlines the importance of understanding fundamental scientific concepts in appreciating the world around us. The simple number 79 holds the key to understanding a fascinating and valuable element.
Latest Posts
Latest Posts
-
What Is The Derivative Of 3x
Mar 16, 2025
-
Lowest Common Multiple Of 6 And 15
Mar 16, 2025
-
Graph Of X 2 Y 2 4
Mar 16, 2025
-
Whats The Square Root Of 196
Mar 16, 2025
-
15 Is What Percent Of 38
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Are In Gold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
