How Many Neutrons Does Carbon 12 Have
listenit
Mar 17, 2025 · 5 min read
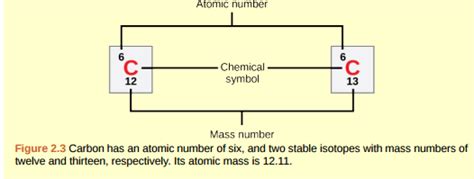
Table of Contents
How Many Neutrons Does Carbon-12 Have? A Deep Dive into Isotopes and Nuclear Structure
Understanding the composition of atoms is fundamental to chemistry and physics. This article delves into the specific question: how many neutrons does carbon-12 have? We'll explore the concept of isotopes, the structure of the carbon atom, and the significance of carbon-12 in various fields, including chemistry, physics, and medicine.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we answer the central question, let's refresh our understanding of atomic structure. Every atom consists of three fundamental subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; all carbon atoms have six protons.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. The number of neutrons can vary within an element, leading to different isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. Electrons are significantly lighter than protons and neutrons.
The atomic number of an element represents the number of protons in its nucleus. Carbon's atomic number is 6, meaning all carbon atoms possess six protons. The mass number, on the other hand, is the sum of protons and neutrons in the nucleus.
Isotopes: Variations in Neutron Count
Isotopes are atoms of the same element that have the same number of protons but differ in their number of neutrons. This difference in neutron count affects the atom's mass but not its chemical properties significantly. Many elements exist naturally as a mixture of isotopes.
Carbon has several isotopes, the most common being:
- Carbon-12 (¹²C): This is the most abundant isotope of carbon, accounting for approximately 99% of all carbon found naturally. It's the standard against which the atomic mass unit (amu) is defined.
- Carbon-13 (¹³C): A stable isotope used in various scientific applications, including carbon dating and nuclear magnetic resonance (NMR) spectroscopy.
- Carbon-14 (¹⁴C): A radioactive isotope used in radiocarbon dating to determine the age of organic materials.
Determining the Number of Neutrons in Carbon-12
Now, we can finally answer the core question: how many neutrons are in carbon-12?
Since the mass number of carbon-12 is 12 and it has 6 protons (its atomic number), the number of neutrons is calculated as follows:
Number of neutrons = Mass number - Atomic number = 12 - 6 = 6
Therefore, carbon-12 has 6 neutrons.
The Significance of Carbon-12
Carbon-12 holds a significant position in several scientific disciplines:
1. Defining the Atomic Mass Unit (amu)
The atomic mass unit (amu), also known as Dalton (Da), is a standard unit for measuring the mass of atoms and molecules. It's defined as one-twelfth the mass of a single carbon-12 atom. This means the mass of a carbon-12 atom is exactly 12 amu.
2. Organic Chemistry and Life
Carbon is the fundamental building block of all organic molecules, forming the backbone of carbohydrates, lipids, proteins, and nucleic acids – the essential components of life. The prevalence of carbon-12 makes it crucial for understanding the chemistry of life.
3. Nuclear Physics and Nuclear Reactions
Carbon-12 plays a crucial role in nuclear reactions. Its stable nucleus is used as a reference point for understanding nuclear forces and interactions. Studying carbon-12 provides insights into the behavior of atomic nuclei, which is vital for areas such as nuclear energy and nuclear medicine.
4. Mass Spectrometry
Mass spectrometry is an analytical technique used to identify and quantify molecules based on their mass-to-charge ratio. Carbon-12's known mass serves as a reference point in mass spectrometry calibrations and analyses. The isotopic ratios of carbon (e.g., the ratio of ¹³C to ¹²C) can provide valuable information about the origin and history of samples.
5. Carbon Dating
While carbon-14 is used for radiocarbon dating, understanding the abundance of carbon-12 is essential for calculating the age of samples accurately. The ratio of carbon-14 to carbon-12 in a sample allows scientists to estimate its age.
Isotopic Abundance and its Implications
The abundance of different isotopes in nature varies depending on the element and its geological history. The relative abundance of carbon isotopes (¹²C, ¹³C, and ¹⁴C) is relatively constant in many natural materials. However, this ratio can change due to various factors, such as metabolic processes in living organisms or specific geological processes.
Variations in isotopic abundance have important implications in various scientific fields:
- Paleoclimatology: Isotopic ratios in ancient ice cores, sediments, and fossils can reveal information about past climate conditions.
- Archaeology: Carbon dating is used to determine the age of artifacts and human remains, providing valuable insights into human history.
- Food Science: Isotopic analysis can be used to track the origin of food products and detect food adulteration.
- Medicine: Stable isotopes are used as tracers in metabolic studies and medical imaging techniques.
Conclusion: The Importance of Understanding Isotopes
In summary, carbon-12 has 6 neutrons. This seemingly simple answer underpins a vast array of scientific understanding and technological applications. By exploring the concept of isotopes and the unique properties of carbon-12, we gain insights into the fundamentals of atomic structure, the chemistry of life, and a range of scientific disciplines. The precise measurement and understanding of isotopic abundances allow scientists to unravel complex processes in the natural world and develop advanced technologies. The significance of carbon-12 extends far beyond its simple atomic composition, highlighting the importance of understanding the subatomic world in its entirety. Further research into isotopic variations continues to reveal even more about our planet's history and the intricacies of the natural world.
Latest Posts
Latest Posts
-
What Is 35 In Fraction Form
Mar 17, 2025
-
What Is 1 20 In Decimal Form
Mar 17, 2025
-
What Is The Square Root Of 39
Mar 17, 2025
-
What Percent Of 400 Is 20
Mar 17, 2025
-
Least Common Multiple Of 4 And 16
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Does Carbon 12 Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
