How Many Lone Pairs In Co2
listenit
Mar 22, 2025 · 5 min read
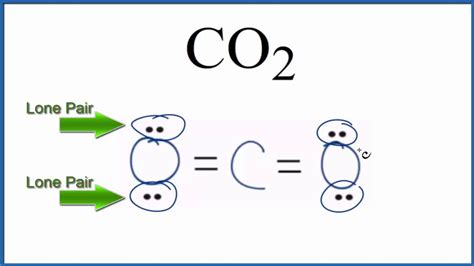
Table of Contents
How Many Lone Pairs in CO₂? A Deep Dive into Carbon Dioxide's Molecular Geometry
Carbon dioxide (CO₂) is a ubiquitous molecule, playing a crucial role in Earth's climate and various industrial processes. Understanding its structure, particularly the number of lone pairs of electrons, is fundamental to comprehending its properties and reactivity. This article will delve into the details of CO₂'s molecular geometry, explaining how many lone pairs are present and why its structure dictates its behavior. We’ll also explore related concepts, providing a comprehensive overview for students and enthusiasts alike.
Understanding Lewis Structures and Valence Electrons
Before we tackle the lone pairs in CO₂, let's review some fundamental concepts. The Lewis structure, also known as an electron dot diagram, is a visual representation of the bonding and non-bonding electrons in a molecule. It helps us predict the molecule's shape and properties. To draw a Lewis structure, we need to know the number of valence electrons each atom possesses.
- Carbon (C): Carbon is in group 14 of the periodic table, meaning it has four valence electrons.
- Oxygen (O): Oxygen is in group 16, possessing six valence electrons.
In CO₂, we have one carbon atom and two oxygen atoms. Therefore, the total number of valence electrons is: (4) + 2(6) = 16 valence electrons.
Constructing the Lewis Structure of CO₂
-
Central Atom: Carbon, being less electronegative than oxygen, is typically placed at the center of the molecule.
-
Connecting Atoms: We connect the carbon atom to each oxygen atom with a single bond, using two electrons per bond. This uses four of our 16 valence electrons.
-
Octet Rule: To satisfy the octet rule (each atom aims to have eight electrons in its valence shell), we distribute the remaining 12 electrons (16 - 4 = 12) around the oxygen atoms. Each oxygen atom receives three lone pairs (6 electrons) to complete its octet.
-
Formal Charges: At this point, the carbon atom only has four electrons surrounding it (two from each single bond), which is short of an octet. To resolve this, we convert one lone pair from each oxygen atom into a double bond with the carbon atom.
The final Lewis structure shows carbon double-bonded to each oxygen atom: O=C=O.
How Many Lone Pairs are in CO₂?
With the Lewis structure complete, we can definitively answer the question: CO₂ has four lone pairs of electrons. Each oxygen atom has two lone pairs (a total of four lone pairs for the entire molecule).
The Linear Geometry of CO₂ and its Implications
The arrangement of atoms and lone pairs influences the molecule's overall geometry. According to the Valence Shell Electron Pair Repulsion (VSEPR) theory, electron pairs, both bonding and non-bonding, repel each other. They arrange themselves to minimize this repulsion, resulting in a specific molecular geometry.
In CO₂, the central carbon atom has two double bonds and no lone pairs. The two double bonds repel each other equally, leading to a linear molecular geometry. The bond angle is 180°. This linear structure significantly impacts CO₂'s properties:
-
Non-polarity: Because the molecule is linear and symmetrical, the individual bond dipoles cancel each other out, resulting in a nonpolar molecule. This impacts its solubility and interactions with other molecules.
-
Weak Intermolecular Forces: The nonpolar nature of CO₂ means it experiences only weak London Dispersion Forces (LDFs) between its molecules. This results in a relatively low boiling point and ease of vaporization.
-
Reactivity: The presence of double bonds makes CO₂ reactive under specific conditions. It can participate in reactions like addition reactions and reduction reactions.
Comparing CO₂ with Other Molecules
To further understand lone pairs and their impact on molecular geometry, let's compare CO₂ with other molecules:
-
Water (H₂O): Water has two bonding pairs and two lone pairs on the central oxygen atom. This results in a bent molecular geometry with a bond angle of approximately 104.5°. Water is a polar molecule due to the lone pairs creating an asymmetrical distribution of charge.
-
Ammonia (NH₃): Ammonia has three bonding pairs and one lone pair on the central nitrogen atom. This results in a trigonal pyramidal geometry. Ammonia is a polar molecule due to the lone pair's influence on the molecular geometry.
-
Methane (CH₄): Methane has four bonding pairs and no lone pairs on the central carbon atom. This results in a tetrahedral geometry and a nonpolar molecule.
Advanced Concepts and Applications
Understanding the lone pairs in CO₂ opens doors to exploring advanced concepts:
-
Hybridization: The carbon atom in CO₂ undergoes sp hybridization, where one s orbital and one p orbital combine to form two sp hybrid orbitals. These sp orbitals form sigma bonds with the oxygen atoms. The remaining p orbitals on carbon and oxygen form pi bonds.
-
Molecular Orbital Theory: A more sophisticated approach to understanding bonding involves molecular orbital theory. This theory considers the combination of atomic orbitals to form molecular orbitals that encompass the entire molecule.
-
Spectroscopy: Techniques like infrared (IR) and Raman spectroscopy can provide experimental evidence confirming the linear geometry and the absence of lone pairs on the carbon atom in CO₂.
Conclusion
In summary, carbon dioxide (CO₂) possesses four lone pairs of electrons, two on each oxygen atom. This arrangement, along with the double bonds between carbon and oxygen, dictates its linear geometry, nonpolar nature, and characteristic properties. Understanding the number and distribution of lone pairs is crucial for predicting a molecule's behavior and reactivity. This knowledge extends beyond simple Lewis structures and encompasses more sophisticated bonding theories and spectroscopic techniques, ultimately contributing to a deeper understanding of chemical systems. The principles outlined in this article can be applied to numerous other molecules, highlighting the importance of lone pairs in determining molecular shape and properties.
Latest Posts
Latest Posts
-
What Is Prime Factorization Of 63
Mar 24, 2025
-
Least Common Multiple 14 And 21
Mar 24, 2025
-
What Is The Lcm Of 8 12
Mar 24, 2025
-
What Percent Is 153 Out Of 392
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How Many Lone Pairs In Co2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
