How Many Electrons Can The S Sublevel Hold
listenit
Mar 30, 2025 · 6 min read
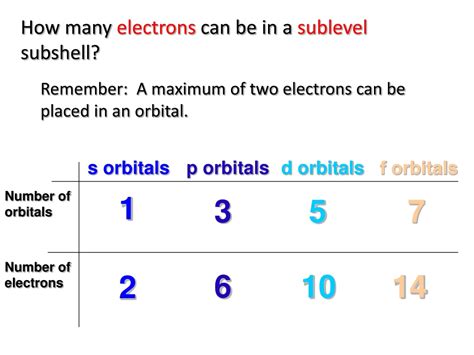
Table of Contents
How Many Electrons Can the s Sublevel Hold? A Deep Dive into Atomic Structure
Understanding the intricacies of atomic structure is fundamental to grasping the principles of chemistry and physics. A crucial aspect of this understanding involves comprehending electron configuration and the capacity of different sublevels within an atom to hold electrons. This article will delve deep into the question: How many electrons can the s sublevel hold? We'll explore the underlying principles of quantum mechanics, the significance of the Pauli Exclusion Principle, and the practical implications of this electron capacity in chemical bonding and reactivity.
Understanding Electron Shells and Sublevels
Before we tackle the specific capacity of the s sublevel, let's establish a foundational understanding of electron shells and sublevels. Atoms are composed of a nucleus containing protons and neutrons, surrounded by electrons orbiting in various energy levels. These energy levels are often referred to as electron shells or principal energy levels, denoted by the principal quantum number (n). The value of 'n' can be any positive integer (1, 2, 3, etc.), with higher values representing shells further from the nucleus and possessing higher energy.
Each electron shell is further subdivided into subshells or sublevels, each characterized by its unique shape and energy. These subshells are denoted by the letters s, p, d, and f. The number of subshells within a given shell is equal to the value of the principal quantum number (n). For instance:
- Shell 1 (n=1): Contains only the s subshell (1s).
- Shell 2 (n=2): Contains the s and p subshells (2s and 2p).
- Shell 3 (n=3): Contains the s, p, and d subshells (3s, 3p, and 3d).
- Shell 4 (n=4): Contains the s, p, d, and f subshells (4s, 4p, 4d, and 4f).
The shapes of these subshells are crucial in determining the spatial distribution of electrons and their interactions. The s subshell is spherical, the p subshell is dumbbell-shaped, and the d and f subshells possess more complex shapes.
The Significance of the Pauli Exclusion Principle
The key to determining the maximum number of electrons a sublevel can hold lies in the Pauli Exclusion Principle. This fundamental principle of quantum mechanics states that no two electrons in an atom can have the same set of four quantum numbers. These four quantum numbers describe the unique state of an electron:
- Principal Quantum Number (n): Describes the electron shell (energy level).
- Azimuthal Quantum Number (l): Describes the subshell (s, p, d, f), with l = 0 for s, l = 1 for p, l = 2 for d, and l = 3 for f.
- Magnetic Quantum Number (ml): Describes the orientation of the orbital within the subshell. For s (l=0), ml = 0; for p (l=1), ml = -1, 0, +1; and so on.
- Spin Quantum Number (ms): Describes the intrinsic angular momentum (spin) of the electron, which can be either +1/2 (spin up) or -1/2 (spin down).
The Pauli Exclusion Principle dictates that each orbital can hold a maximum of two electrons, one with spin up (+1/2) and one with spin down (-1/2).
How Many Electrons Can the s Sublevel Hold?
Now, let's focus on the s sublevel. The s subshell has only one orbital (ml = 0). Since each orbital can hold a maximum of two electrons (according to the Pauli Exclusion Principle), the s sublevel can hold a maximum of two electrons. This holds true for all s subshells, regardless of the principal quantum number (1s, 2s, 3s, etc.).
Therefore, the answer is two.
Implications of the s Sublevel's Electron Capacity
The fact that the s sublevel can only hold two electrons has significant implications in various areas of chemistry and physics:
1. Chemical Bonding:
The electrons in the outermost s sublevel (valence electrons) play a crucial role in chemical bonding. Elements with a completely filled s subshell (like helium or the alkaline earth metals in their ground state) tend to be less reactive than elements with partially filled s subshells. The capacity of the s subshell directly influences the number of bonds an atom can form.
2. Periodic Trends:
The filling of the s subshell influences the periodic trends observed in the periodic table. For example, the alkali metals (Group 1) have one electron in their outermost s subshell, leading to their high reactivity. The alkaline earth metals (Group 2) have two electrons in their outermost s subshell, making them less reactive than the alkali metals.
3. Atomic Radii:
The number of electrons in the s subshell influences the size of an atom. As we move down a group in the periodic table, the addition of electrons to higher energy levels (including s subshells) leads to an increase in atomic radius.
4. Ionization Energy:
The energy required to remove an electron from an atom (ionization energy) is also influenced by the electron configuration, particularly the filling of the s sublevel. Elements with completely filled s subshells tend to have higher ionization energies than elements with partially filled s subshells.
Beyond the s Sublevel: Electron Configuration and Orbital Filling
While we've focused on the s sublevel, understanding the electron capacity of other subshells is equally important for comprehending atomic structure. The p subshell, with three orbitals, can hold up to six electrons; the d subshell, with five orbitals, can hold up to ten electrons; and the f subshell, with seven orbitals, can hold up to fourteen electrons.
The filling of these subshells follows specific rules, often summarized by the Aufbau principle (filling orbitals in order of increasing energy) and Hund's rule (maximizing the number of unpaired electrons with parallel spins before pairing them). These principles, in conjunction with the Pauli Exclusion Principle, govern the electron configurations of all atoms.
Advanced Concepts and Applications
The seemingly simple question of how many electrons an s sublevel can hold opens doors to more complex concepts:
- Quantum numbers and their significance: Deeper exploration into the physical meaning and implications of quantum numbers provides a more nuanced understanding of electron behavior.
- Electron-electron repulsion: The interactions between electrons within the same sublevel impact their energy levels and affect the overall stability of the atom.
- Hybridization: The concept of orbital hybridization explains how atomic orbitals combine to form new hybrid orbitals, influencing molecular geometry and bonding properties. This process often involves s orbitals.
- Spectroscopy: The study of how atoms interact with electromagnetic radiation provides experimental evidence to support our understanding of electron configurations and energy levels.
Conclusion
The seemingly straightforward question—how many electrons can the s sublevel hold?—serves as a gateway to a rich understanding of atomic structure and quantum mechanics. The answer, two electrons, is a direct consequence of the fundamental Pauli Exclusion Principle, and this capacity has profound implications for chemical bonding, periodic trends, and various other atomic properties. By understanding this foundational principle, we unlock the key to understanding the behavior of matter at the atomic level and its macroscopic consequences. The capacity of the s subshell is not just a number; it is a cornerstone of chemical understanding.
Latest Posts
Latest Posts
-
Which Quadrilaterals Always Have Opposite Angles That Are Congruent
Apr 01, 2025
-
What Organelle Carries Out Cellular Respiration
Apr 01, 2025
-
How Many Right Angles Can A Trapezoid Have
Apr 01, 2025
-
What Is 6 8 As A Percent
Apr 01, 2025
-
What Are The Rungs Of Dna Made Of
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The S Sublevel Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
