How Many Electrons Can Each Ring Hold
listenit
Mar 17, 2025 · 6 min read
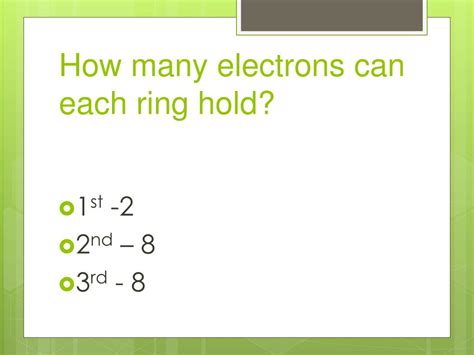
Table of Contents
How Many Electrons Can Each Ring Hold? Understanding Electron Shells and Subshells
Understanding electron configuration is fundamental to comprehending the behavior of atoms and the periodic table. A key aspect of this is knowing how many electrons each electron shell (or energy level) can hold. This article delves deep into the topic, explaining not only the maximum number of electrons per shell but also the intricacies of subshells and the underlying quantum mechanics that govern electron arrangement.
Electron Shells: Energy Levels Around the Nucleus
Atoms consist of a central nucleus containing protons and neutrons, surrounded by orbiting electrons. These electrons don't orbit randomly; they exist in specific energy levels, often visualized as concentric shells or rings around the nucleus. Each shell represents a principal energy level, denoted by the principal quantum number, n. The further away a shell is from the nucleus, the higher its energy level.
- Shell 1 (n=1): This is the shell closest to the nucleus and has the lowest energy.
- Shell 2 (n=2): Further from the nucleus than shell 1, with higher energy.
- Shell 3 (n=3): Even further out, and so on.
The number of electrons a shell can hold is crucial for understanding an atom's stability and reactivity. It's not simply a matter of progressively larger shells holding more electrons; the arrangement follows specific rules dictated by quantum mechanics.
The Maximum Capacity: The 2n² Rule
The maximum number of electrons that can occupy a given shell is determined by the formula 2n², where 'n' is the principal quantum number of the shell. Let's break this down:
- Shell 1 (n=1): 2(1)² = 2 electrons
- Shell 2 (n=2): 2(2)² = 8 electrons
- Shell 3 (n=3): 2(3)² = 18 electrons
- Shell 4 (n=4): 2(4)² = 32 electrons
- Shell 5 (n=5): 2(5)² = 50 electrons
- Shell 6 (n=6): 2(6)² = 72 electrons
- Shell 7 (n=7): 2(7)² = 98 electrons
This formula provides the overall capacity of a shell, but it doesn't tell the whole story. Electrons within a shell are further organized into subshells.
Subshells: Diving Deeper into Electron Arrangement
Each electron shell is further divided into subshells, which are regions of space within the shell where electrons are more likely to be found. These subshells are designated by letters: s, p, d, and f.
- s subshell: This is the lowest energy subshell within a shell and can hold a maximum of 2 electrons.
- p subshell: Higher in energy than the s subshell and can hold a maximum of 6 electrons.
- d subshell: Higher in energy than the p subshell and can hold a maximum of 10 electrons.
- f subshell: The highest energy subshell within a shell and can hold a maximum of 14 electrons.
The number of subshells within a shell corresponds to the principal quantum number (n). For example:
- Shell 1 (n=1): Contains only the 1s subshell.
- Shell 2 (n=2): Contains the 2s and 2p subshells.
- Shell 3 (n=3): Contains the 3s, 3p, and 3d subshells.
- Shell 4 (n=4): Contains the 4s, 4p, 4d, and 4f subshells.
It's important to note that the filling of subshells follows a specific order, which is not strictly sequential based on the shell number. This order is determined by the Aufbau principle and Hund's rule, topics we'll explore later.
The Aufbau Principle and Hund's Rule: Filling the Subshells
The Aufbau principle states that electrons fill the lowest energy levels first. While shells are arranged radially, the energy levels of subshells overlap. This means the 4s subshell fills before the 3d subshell, for instance. This is why the 2n² rule provides the total capacity, but not the filling order.
Hund's rule explains how electrons fill orbitals within a subshell. Orbitals are regions within a subshell that can hold a maximum of two electrons with opposite spins. Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to greater stability.
Electron Configuration and the Periodic Table
The periodic table is organized based on the electron configurations of elements. The arrangement of elements reflects the sequential filling of electron shells and subshells. Each column (group) represents elements with similar outermost electron configurations, explaining their similar chemical properties.
For example, elements in Group 1 (alkali metals) all have one electron in their outermost s subshell. Elements in Group 18 (noble gases) have completely filled outermost shells, making them exceptionally stable and unreactive.
Exceptions to the Rules: Why Some Atoms Don't Follow the Expected Pattern
While the Aufbau principle and Hund's rule provide a framework for predicting electron configurations, some exceptions exist. These exceptions often involve transition metals and some heavier elements. The deviations stem from subtle energy differences between subshells and the influence of electron-electron interactions. These exceptions don't invalidate the general principles but highlight the complexities of electron behavior in atoms with many electrons.
Beyond the Basics: Orbital Shapes and Quantum Numbers
The description of electron shells and subshells above simplifies a more complex reality. Each subshell contains one or more orbitals, and each orbital has a specific shape and orientation in space. These orbitals are described by quantum numbers:
- Principal quantum number (n): Determines the energy level and shell number.
- Azimuthal quantum number (l): Specifies the subshell (s, p, d, f) and the shape of the orbital.
- Magnetic quantum number (ml): Defines the orientation of the orbital in space.
- Spin quantum number (ms): Describes the intrinsic angular momentum of the electron (spin up or spin down).
The combination of these quantum numbers uniquely specifies the state of an electron in an atom.
Practical Applications: Understanding Chemical Bonding and Reactivity
Understanding electron configuration is critical for understanding chemical bonding and reactivity. Atoms tend to react in ways that achieve a more stable electron configuration, often by filling their outermost shell (the valence shell). This drive for stability is the basis of many chemical reactions and the formation of chemical compounds.
Conclusion: A Deeper Understanding of Atomic Structure
This in-depth exploration of how many electrons each ring (shell) can hold reveals the elegance and complexity of atomic structure. While the 2n² rule provides a concise overview of the maximum electron capacity of each shell, the finer details of subshells, orbitals, and quantum mechanics are essential for a complete understanding. This knowledge is foundational to chemistry, physics, and materials science, impacting our understanding of the world at the atomic level. The principles discussed here underpin our understanding of chemical bonding, reactivity, and the properties of matter. By mastering these concepts, one can gain a deeper appreciation for the intricate workings of the universe at its most fundamental level. Further exploration into quantum mechanics will provide even greater insight into the subtleties and complexities of electron behavior.
Latest Posts
Latest Posts
-
What Determines The Shape Of A Protein
Mar 18, 2025
-
How Much Is 70 Degrees Fahrenheit In Celsius
Mar 18, 2025
-
24 Is What Percent Of 120
Mar 18, 2025
-
A Quadrilateral With Two Pairs Of Parallel Sides
Mar 18, 2025
-
1 4 To The Power Of 2
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can Each Ring Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
