How Many Electrons Are In An Atom Of Copper
listenit
Mar 20, 2025 · 6 min read
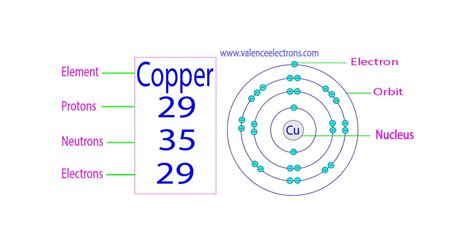
Table of Contents
How Many Electrons Are in an Atom of Copper? Delving into Atomic Structure and Electron Configuration
Copper, a reddish-orange metal known for its excellent electrical conductivity and malleability, plays a crucial role in various applications, from electrical wiring to plumbing. Understanding its atomic structure, specifically the number of electrons it possesses, is fundamental to grasping its properties and behavior. So, how many electrons are in an atom of copper? The answer, simply put, is 29. But this simple answer opens the door to a deeper exploration of atomic structure, electron configuration, and the fascinating world of quantum mechanics.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we dive into copper's electron count, let's establish a foundational understanding of atomic structure. An atom, the fundamental building block of matter, consists of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element; it's the atomic number.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons in a neutral atom equals the number of protons.
In a neutral atom, the positive charge of the protons is balanced by the negative charge of the electrons, resulting in a net charge of zero. This balance is crucial for the atom's stability.
Copper's Atomic Number and Electron Configuration
Copper (Cu) has an atomic number of 29. This means a neutral copper atom contains 29 protons in its nucleus. Consequently, a neutral copper atom also contains 29 electrons.
However, simply stating that copper has 29 electrons doesn't tell the whole story. These electrons are not randomly distributed around the nucleus. They occupy specific energy levels or shells, following the principles of quantum mechanics and the Aufbau principle. This organized arrangement is called the electron configuration.
The electron configuration of copper is typically written as: 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰.
Let's break down this notation:
- 1s²: The first energy level (n=1) contains one subshell (s) with two electrons.
- 2s²2p⁶: The second energy level (n=2) contains two subshells: an s subshell with two electrons and a p subshell with six electrons.
- 3s²3p⁶: The third energy level (n=3) also contains an s subshell with two electrons and a p subshell with six electrons.
- 4s¹3d¹⁰: This is where things get interesting. The fourth energy level (n=4) starts filling, with one electron in the s subshell. However, instead of continuing to fill the 4p subshell, the 3d subshell, which is slightly lower in energy, fills completely with ten electrons.
This exception to the typical Aufbau principle filling order is due to the stability gained by having a completely filled 3d subshell. A completely filled or half-filled subshell is energetically more favorable, contributing to copper's unique properties.
The Significance of Copper's Electron Configuration and its Properties
Copper's electron configuration directly influences its physical and chemical properties:
-
Electrical Conductivity: The loosely held electron in the 4s subshell is readily available to participate in electrical conduction. This electron, along with the relatively delocalized d-electrons, allows for efficient movement of charge, making copper an excellent conductor of electricity.
-
Thermal Conductivity: Similar to electrical conductivity, the mobile electrons in copper contribute significantly to its high thermal conductivity. Heat is transferred efficiently through the movement of these electrons.
-
Malleability and Ductility: The metallic bonding in copper, resulting from the interaction of its valence electrons, allows its atoms to slide past each other without breaking the metallic bonds. This makes copper easily malleable (can be hammered into shapes) and ductile (can be drawn into wires).
-
Reddish-Orange Color: The interaction of light with the electrons in copper's d-orbitals results in the absorption and reflection of specific wavelengths of light, giving copper its characteristic reddish-orange color.
Isotopes of Copper and Electron Number
While the number of electrons in a neutral copper atom is always 29, it's important to note the existence of copper isotopes. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. The most common isotopes of copper are copper-63 (⁶³Cu) and copper-65 (⁶⁵Cu).
Both ⁶³Cu and ⁶⁵Cu have 29 protons and 29 electrons in their neutral state. The difference lies in the number of neutrons: ⁶³Cu has 34 neutrons, while ⁶⁵Cu has 36 neutrons. The different number of neutrons affects the atom's mass but not its chemical properties significantly because the chemical properties are primarily determined by the number of electrons and their arrangement.
Copper's Role in Biology and Technology
Copper's unique properties have led to its widespread use in various applications:
- Electrical Wiring: Copper's excellent electrical conductivity makes it essential for electrical wiring in homes, buildings, and power grids.
- Plumbing: Copper pipes are widely used in plumbing systems due to their resistance to corrosion and durability.
- Coins: Copper is a component of many coins, contributing to their durability and aesthetic appeal.
- Industrial Catalysts: Copper compounds are utilized as catalysts in various industrial processes.
- Biological Roles: Copper plays an essential role in many biological processes, serving as a cofactor in several enzymes.
Beyond the Basics: Advanced Concepts
The simple answer of 29 electrons in a copper atom opens up the possibility to delve into more advanced concepts:
-
Quantum Numbers: Each electron in a copper atom can be described by four quantum numbers: principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). These numbers specify the electron's energy level, subshell, orbital, and spin.
-
Molecular Orbitals: When copper atoms bond with other atoms to form molecules or solids, their atomic orbitals combine to form molecular orbitals. Understanding molecular orbitals is crucial for understanding the properties of copper compounds.
-
Spectroscopy: Analyzing the interaction of light with copper atoms allows us to gain insights into their electronic structure. Spectroscopic techniques provide valuable information about the energy levels and transitions of electrons within the copper atom.
-
Nuclear Physics: While the focus here has been on the electrons, exploring the nucleus of copper, its isotopes, and potential nuclear reactions expands the understanding even further.
Conclusion
While the answer to the question "How many electrons are in an atom of copper?" is simply 29, understanding this number requires a deeper dive into the fascinating world of atomic structure, electron configuration, and quantum mechanics. Copper's 29 electrons, arranged according to its unique electron configuration, are responsible for its remarkable electrical conductivity, thermal conductivity, malleability, and other properties that make it such a valuable material in diverse applications, from everyday life to high-tech industries. Further exploration of copper's atomic structure unveils a wealth of knowledge related to its chemical behavior, biological roles, and technological applications. The seemingly simple number 29, therefore, represents a gateway to a vast and intricate understanding of the material world.
Latest Posts
Latest Posts
-
What Is 25 In Fraction Form
Mar 21, 2025
-
Which Plane Divides The Body Into Left And Right Sides
Mar 21, 2025
-
11 Out Of 15 In Percentage
Mar 21, 2025
-
How Many Protons Does Magensiuum Ion Have
Mar 21, 2025
-
Domain And Range Of Arcsin X
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In An Atom Of Copper . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
