How Many Bonds Does Boron Form
listenit
Mar 23, 2025 · 6 min read
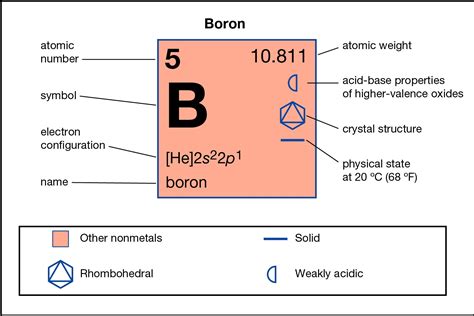
Table of Contents
How Many Bonds Does Boron Form? Understanding Boron's Bonding Behavior
Boron, the fifth element on the periodic table, presents a fascinating case study in chemical bonding. Unlike its neighbors carbon and aluminum, boron's bonding behavior isn't easily categorized. While a simplistic answer might point to three bonds, the reality is far more nuanced and depends on several factors, including the surrounding atoms, the overall molecular structure, and the presence of electron deficiency. This article delves deep into the intricacies of boron bonding, exploring its ability to form multiple bonds and the reasons behind its unique characteristics.
The Simple Answer: Three Bonds
At a basic level, introductory chemistry often presents boron as forming three covalent bonds. This arises from its electronic configuration: 1s²2s²2p¹. Boron has three valence electrons in its 2s and 2p orbitals, seemingly capable of sharing these three electrons to achieve a stable octet (eight valence electrons). This is apparent in simple molecules like borane (BH₃), although its existence as a stable monomer is rather limited due to its electron-deficient nature.
The Octet Rule and its Limitations with Boron
The octet rule, a guideline stating that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight valence electrons, serves as a useful simplification. However, it is crucial to remember that it's not a hard and fast law. Boron, with its electron-deficient nature, frequently violates the octet rule. The resulting structures exhibit unique properties and reactivity.
The Reality: Beyond Three Bonds – Electron Deficiency and Hypervalency
The tendency of boron to form fewer than eight bonds leads to electron deficiency. This means the boron atom in certain compounds doesn't have a full octet of electrons in its valence shell. This electron deficiency is the key to understanding why boron sometimes forms more, or even fewer, than three bonds. The behavior often deviates from the straightforward three-bond model:
- Hypervalency in boron compounds is rare but possible: While not as common as in other elements like phosphorus or sulfur, boron can sometimes exhibit hypervalency, exceeding the expected number of bonds. This usually occurs in situations where the overall molecular structure allows for additional bonding interactions to occur. The presence of highly electronegative atoms can help stabilize such structures. It's important to note that the term "hypervalent" is sometimes debated for boron, owing to the differing ways bonding can be described, but we'll use the term here to signify exceeding the typical three bonds.
- Dative Bonding: Boron frequently participates in dative (coordinate covalent) bonds. This happens when one atom provides both electrons for a shared pair in a bond. This allows boron to effectively increase its coordination number (the number of atoms bonded to it), beyond what its valence electrons alone would suggest. This feature is very common in boron's chemistry.
Factors influencing Boron's Bonding Number
Several factors influence the precise number of bonds a boron atom will form in a given molecule or compound. These factors intertwine to dictate the final structure and its properties:
1. Electronegativity of the Bonded Atoms
The electronegativity of the atoms bonded to boron strongly influences its bonding behavior. Highly electronegative atoms such as fluorine, oxygen, and nitrogen can effectively draw electron density away from the boron atom, stabilizing electron-deficient structures and allowing for greater stability in the resulting compound. In other words, these atoms assist in stabilizing hypervalent boron, albeit unusually.
2. Steric Effects
The spatial arrangement of atoms (steric effects) plays a critical role. Bulky substituents around the boron atom may hinder the formation of additional bonds, favoring structures with fewer bonds. Conversely, less sterically demanding substituents allow for increased coordination.
3. Molecular Structure and Bonding
The overall molecular structure significantly affects the number of bonds boron forms. The structural requirements of larger molecular frameworks can necessitate more than three bonds, pushing boron towards hypervalency or increased coordination number. The need to satisfy overall molecular stability drives this complex bond formation.
4. Presence of other ligands
The nature and number of ligands (atoms or groups attached to the boron atom) directly influence the bonding behavior. Different ligands can alter the electron density around boron, impacting its ability to form additional bonds and potentially stabilizing electron-deficient structures.
Examples of Boron's Variable Bonding
Let's examine some examples showcasing boron's diverse bonding capabilities:
1. Boron Trifluoride (BF₃)
BF₃ is a classic example of a compound where boron forms three covalent bonds. However, the molecule is electron-deficient, and BF₃ readily acts as a Lewis acid, accepting a lone pair of electrons from a Lewis base to form a stable adduct with four bonds.
2. Boranes (BH₃) and Borane Adducts
BH₃ (borane) itself is an unstable molecule. Due to its electron-deficient nature, it's often found as a dimer (B₂H₆) or in adducts with Lewis bases. This dimerization and adduct formation highlight its need for more electrons to achieve greater stability.
3. Tetrahedral Boron Complexes
In some organometallic complexes, boron forms four bonds, adopting a tetrahedral geometry. These structures involve dative bonding or additional electron donation from ligands to stabilize the boron atom.
4. Boron-Nitrogen Compounds
Boron readily forms bonds with nitrogen, forming a stable bond. These compounds can exhibit various bonding patterns, sometimes involving more than three bonds to boron, stabilizing otherwise electron-deficient structures. These bonds are often strong and essential in many chemical processes.
Analytical Techniques for Determining Boron Bonding
Determining the precise number of bonds boron forms in a specific compound relies on several analytical techniques:
- X-ray Crystallography: This technique provides a detailed picture of the molecular structure, allowing direct visualization of the number of bonds.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy can provide valuable information on the bonding environment of boron atoms, indirectly revealing the number of bonds.
- Computational Chemistry: Advanced computational methods can be used to model and predict the bonding in boron compounds, offering insights into the bonding configurations and energies.
Conclusion: A Complex and Versatile Element
In conclusion, while boron is often introduced as forming three bonds, the reality is far richer and more intricate. Its electron deficiency significantly influences its bonding behavior, leading to a variable number of bonds. The actual number of bonds formed depends intricately on the surrounding atoms, molecular structure, and presence of electron-donating ligands. Boron's diverse bonding behavior makes it a vital component in a vast array of compounds with diverse applications, showcasing its unique and important role in chemistry. Understanding its variable bonding is essential for comprehending the properties and reactivity of many boron-containing materials. Ongoing research continues to unveil the complexities of boron's fascinating bonding behavior.
Latest Posts
Latest Posts
-
How Many Lbs Is 1 3 Kg
Mar 24, 2025
-
What Is The Decimal Of 5 12
Mar 24, 2025
-
Protons Neutrons And Electrons In Sodium
Mar 24, 2025
-
Write The Chemical Equation For Cellular Respiration
Mar 24, 2025
-
What Is The Lcm For 7 And 10
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How Many Bonds Does Boron Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
