How Many Atoms Are In H20
listenit
Mar 29, 2025 · 5 min read
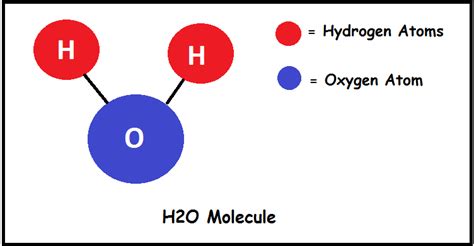
Table of Contents
How Many Atoms Are in H₂O? A Deep Dive into Molecular Composition
Water, the elixir of life, is a deceptively simple molecule with a profound impact on our world. Its chemical formula, H₂O, tells us that it's composed of hydrogen and oxygen, but understanding how many atoms are present in a single water molecule, and then scaling that up to larger quantities, requires a closer look at atomic structure and stoichiometry.
Understanding the Basics: Atoms and Molecules
Before delving into the specifics of water, let's establish a foundational understanding of atoms and molecules.
Atoms: The Building Blocks of Matter
Atoms are the fundamental units of matter. They are incredibly small particles, consisting of a dense central nucleus containing protons (positively charged) and neutrons (neutral), surrounded by a cloud of electrons (negatively charged). The number of protons in an atom's nucleus defines its atomic number and determines which element it is. For example, hydrogen (H) has an atomic number of 1 (one proton), while oxygen (O) has an atomic number of 8 (eight protons).
Molecules: Combining Atoms
When atoms bond together, they form molecules. These bonds arise from the interactions between the electrons of the constituent atoms. In a water molecule (H₂O), two hydrogen atoms are covalently bonded to a single oxygen atom. A covalent bond involves the sharing of electrons between atoms. This sharing allows the atoms to achieve a more stable electron configuration.
Counting Atoms in H₂O: The Simple Answer
The chemical formula H₂O directly tells us the number of atoms present. The subscript "2" after the H indicates that there are two hydrogen atoms. There's no subscript after the O, implying that there is one oxygen atom. Therefore, a single water molecule contains a total of three atoms: two hydrogen atoms and one oxygen atom.
Scaling Up: From Molecules to Moles
While knowing the number of atoms in a single water molecule is helpful, it's often necessary to work with larger quantities. This is where the concept of a mole comes into play.
The Mole: A Chemist's Dozen
A mole (mol) is a unit of measurement in chemistry that represents a specific number of particles – Avogadro's number, which is approximately 6.022 x 10²³. This immense number is comparable to the number of grains of sand on all the beaches on Earth! One mole of any substance contains Avogadro's number of particles, whether those particles are atoms, molecules, ions, or anything else.
Moles and Water: Putting it Together
Since one water molecule (H₂O) contains three atoms, one mole of water contains three times Avogadro's number of atoms. That's:
3 atoms/molecule * 6.022 x 10²³ molecules/mol = 1.807 x 10²⁴ atoms/mol
This means that one mole of water contains approximately 1.807 x 10²⁴ atoms.
Exploring Different Quantities of Water: Practical Applications
Let's explore how we can apply this knowledge to different quantities of water.
A Glass of Water: How Many Atoms?
A typical glass of water contains approximately 250 milliliters (mL) of water. Since the density of water is approximately 1 gram per milliliter (g/mL), this glass contains about 250 grams of water.
To determine the number of atoms, we need to convert grams to moles using the molar mass of water. The molar mass of water (H₂O) is approximately 18 grams per mole (g/mol) (1g/mol for H * 2 + 16 g/mol for O).
Therefore:
250 g / 18 g/mol ≈ 13.9 moles of water
Using the calculation from above:
13.9 mol * 1.807 x 10²⁴ atoms/mol ≈ 2.51 x 10²⁵ atoms
This means a typical glass of water contains approximately 2.51 x 10²⁵ atoms!
An Ocean's Worth of Atoms: An Immense Scale
Scaling this up to the vastness of an ocean is truly mind-boggling. The estimated volume of water in the Earth's oceans is around 1.332 billion cubic kilometers. Converting this to grams and then moles, and subsequently to atoms, would yield an astronomically large number – far beyond comprehension. However, the principle remains the same: the number of atoms would be the number of moles multiplied by 1.807 x 10²⁴ atoms/mol.
Isotopes and Atomic Mass: A Deeper Dive
The discussion above assumes a simplified model of water molecules composed of the most common isotopes of hydrogen and oxygen. However, isotopes exist for both elements. Isotopes are atoms of the same element that have different numbers of neutrons. This means that their atomic masses differ slightly.
Hydrogen Isotopes: Protium, Deuterium, and Tritium
Hydrogen has three main isotopes: protium (¹H), deuterium (²H), and tritium (³H). Protium is the most abundant and the one typically considered in basic calculations. Deuterium and tritium have one and two extra neutrons, respectively, affecting the water molecule's mass.
Oxygen Isotopes: Variations in Abundance
Oxygen also has several isotopes, the most common being ¹⁶O, ¹⁷O, and ¹⁸O. The presence of these isotopes affects the average molar mass of water and consequently the total number of atoms calculated.
While the effect on the total atom count from the less abundant isotopes isn’t significant in basic calculations, it highlights the complexity present at the atomic level. Precise calculations require taking these isotopic variations into account, resulting in slightly varying estimations of the total number of atoms.
Conclusion: The Astonishing Scale of Atoms
From a single molecule to the vast expanse of the oceans, the sheer number of atoms present in even relatively small quantities of water is astonishing. While calculating the exact number requires understanding moles, molar mass, and considering isotopic variations, the core concept remains consistent: each water molecule contains three atoms – two hydrogen and one oxygen – and the total number of atoms scales proportionally with the quantity of water. This fundamental understanding provides insights into the incredible scale of the atomic world and the vastness of even seemingly simple substances like water. The principles discussed here form the bedrock of many advanced concepts in chemistry and physics, revealing the underlying structure of the matter around us.
Latest Posts
Latest Posts
-
What Percent Of 25 Is 4
Mar 31, 2025
-
Number Of Protons Neutrons And Electrons In Carbon
Mar 31, 2025
-
What Percentage Is 4 Out Of 20
Mar 31, 2025
-
The Mercalli Scale Is A Scale From
Mar 31, 2025
-
Is Iodine A Metal Metalloid Or Nonmetal
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In H20 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
