How Is Element Different From Compound
listenit
Mar 21, 2025 · 7 min read
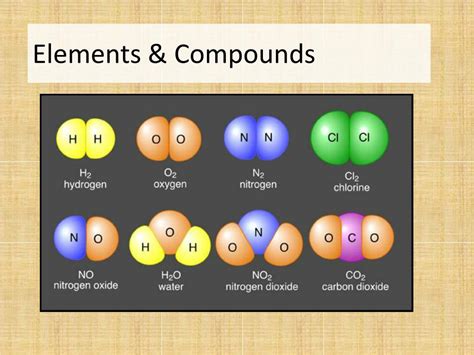
Table of Contents
How is an Element Different from a Compound? A Deep Dive into Matter
Understanding the fundamental building blocks of matter is crucial in chemistry and many related scientific fields. At the heart of this understanding lies the distinction between elements and compounds. While both are forms of matter, their composition and properties differ significantly. This article delves deep into the differences between elements and compounds, exploring their definitions, characteristics, and examples. We will also touch upon the concepts of mixtures, further clarifying the classification of matter.
What is an Element?
An element is a pure substance consisting entirely of one type of atom. Atoms are the smallest units of matter that retain the chemical properties of an element. Each element is defined by the number of protons in its atomic nucleus, known as its atomic number. This number uniquely identifies the element and determines its position on the periodic table. For instance, hydrogen (H) has an atomic number of 1, meaning each hydrogen atom possesses one proton. Oxygen (O) has an atomic number of 8, and gold (Au) has an atomic number of 79.
Key Characteristics of Elements:
- Pure Substance: Elements are not mixed with any other substance. They are composed solely of atoms with the same atomic number.
- Unique Properties: Each element possesses a unique set of chemical and physical properties, including melting point, boiling point, density, reactivity, and conductivity. These properties are determined by the element's atomic structure, specifically the number of protons, neutrons, and electrons.
- Cannot be Broken Down: Elements cannot be broken down into simpler substances by chemical means. While nuclear reactions can alter the composition of an element's nucleus (e.g., nuclear fission or fusion), chemical reactions cannot.
- Represented by Symbols: Elements are represented by one or two-letter symbols, usually derived from their English or Latin names. For example, H for hydrogen, O for oxygen, Fe for iron (from the Latin ferrum), and Au for gold (from the Latin aurum).
What is a Compound?
A compound is a pure substance formed when two or more different elements are chemically bonded together in a fixed ratio. This bonding occurs through the sharing or transfer of electrons between atoms, creating a new substance with properties distinct from its constituent elements. The ratio of elements in a compound is always consistent and is represented by its chemical formula.
Key Characteristics of Compounds:
- Chemical Combination: Compounds are formed through chemical reactions that involve the rearrangement of atoms. The atoms in a compound are held together by strong chemical bonds, such as covalent or ionic bonds.
- Fixed Ratio: The elements in a compound are always present in a fixed, definite proportion by mass. This is known as the Law of Definite Proportions. For example, water (H₂O) always contains two hydrogen atoms for every one oxygen atom.
- Different Properties than Constituent Elements: The properties of a compound are vastly different from the properties of the elements that compose it. For instance, sodium (Na) is a highly reactive metal, and chlorine (Cl) is a toxic gas. However, their combination forms sodium chloride (NaCl), or common table salt, a relatively inert, crystalline solid.
- Can be Broken Down: Compounds can be broken down into simpler substances (the constituent elements) through chemical reactions. This can be achieved through various methods like electrolysis or chemical decomposition.
- Represented by Chemical Formulas: Compounds are represented by chemical formulas that show the types and numbers of atoms present in one molecule or formula unit of the compound. For example, H₂O for water, NaCl for sodium chloride, and C₆H₁₂O₆ for glucose.
The Crucial Differences: A Comparison Table
| Feature | Element | Compound |
|---|---|---|
| Composition | One type of atom | Two or more different types of atoms |
| Bonding | No chemical bonds between atoms (except in certain allotropes) | Chemical bonds between atoms (covalent or ionic) |
| Properties | Unique properties; determined by atomic number | Properties different from constituent elements |
| Breakdown | Cannot be broken down chemically | Can be broken down chemically |
| Ratio | N/A | Fixed ratio of elements |
| Examples | Oxygen (O), Iron (Fe), Gold (Au) | Water (H₂O), Salt (NaCl), Glucose (C₆H₁₂O₆) |
Beyond Elements and Compounds: Understanding Mixtures
While elements and compounds are pure substances, mixtures are combinations of two or more substances that are not chemically bonded. Mixtures can be homogeneous (uniform composition throughout, like saltwater) or heterogeneous (non-uniform composition, like sand and water). The components of a mixture retain their individual properties and can be separated by physical methods, such as filtration, distillation, or evaporation. This is a key difference from compounds, where the components lose their original properties and require chemical reactions for separation.
Examples Illustrating the Differences
Let's consider some specific examples to further clarify the distinction:
-
Water (H₂O): Water is a compound because it is formed by the chemical bonding of two hydrogen atoms and one oxygen atom. The properties of water are vastly different from those of hydrogen and oxygen. Hydrogen is a flammable gas, and oxygen supports combustion. Water, however, is a liquid at room temperature and extinguishes flames.
-
Salt (NaCl): Sodium chloride, or table salt, is a compound formed from the ionic bonding of sodium (Na) and chlorine (Cl) atoms. Sodium is a highly reactive metal, and chlorine is a toxic gas. However, their combination results in a crystalline solid, sodium chloride, which is essential for human life.
-
Air: Air is a mixture of various gases, primarily nitrogen (N₂) and oxygen (O₂), along with smaller amounts of argon (Ar), carbon dioxide (CO₂), and other gases. These gases are not chemically bonded; they simply exist together. Air can be separated into its components through physical processes like fractional distillation.
-
Brass: Brass is an alloy, a type of mixture, made primarily of copper (Cu) and zinc (Zn). The properties of brass differ from those of pure copper and zinc, but the constituent elements remain chemically unchanged.
The Importance of Understanding the Difference
The distinction between elements and compounds is fundamental to understanding chemistry and its applications. Knowing whether a substance is an element or a compound helps us predict its properties, understand its reactivity, and determine how it can be manipulated or used. This knowledge is critical in various fields, including material science, medicine, engineering, and environmental science. For example, understanding the chemical composition of materials is vital for designing new materials with specific properties, developing new medicines and treatments, and assessing the environmental impact of different substances.
Advanced Concepts: Isotopes and Allotropes
To further enrich our understanding, let's briefly touch upon isotopes and allotropes. While they don’t change the fundamental distinction between elements and compounds, they add complexity to the picture.
Isotopes: Atoms of the same element can have different numbers of neutrons, leading to isotopes. While isotopes of the same element have the same number of protons and therefore the same chemical properties, their physical properties (like mass) can differ slightly. For example, carbon-12 and carbon-14 are both isotopes of carbon but have different numbers of neutrons.
Allotropes: Allotropes are different structural modifications of the same element. These variations arise from the different ways atoms of the same element can bond together. For example, carbon exists in different allotropic forms, including diamond, graphite, and fullerenes. While all are composed solely of carbon atoms, they possess vastly different physical and some chemical properties due to their different bonding structures.
Conclusion
The distinction between elements and compounds forms a cornerstone of our understanding of matter. Elements, the fundamental building blocks, are pure substances made up of one type of atom, while compounds are formed from the chemical combination of two or more different elements in a fixed ratio. Understanding this distinction, along with the concept of mixtures, is crucial for grasping the complexities of chemistry and its multifaceted applications across numerous scientific disciplines. This knowledge allows us to predict the behavior of substances, design new materials, and develop innovative solutions to various challenges facing society. By appreciating the fundamental differences between elements and compounds, we gain a deeper appreciation for the intricacies of the matter that constitutes our world.
Latest Posts
Latest Posts
-
What Is The Electron Configuration For Cobalt
Mar 22, 2025
-
Taylor Series For Log 1 X
Mar 22, 2025
-
Why Chemical Equations Have To Be Balanced
Mar 22, 2025
-
Whats The Absolute Value Of 5
Mar 22, 2025
-
Whats The Thinnest Layer Of The Earth
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Is Element Different From Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
