What Is The Electron Configuration For Cobalt
listenit
Mar 22, 2025 · 5 min read
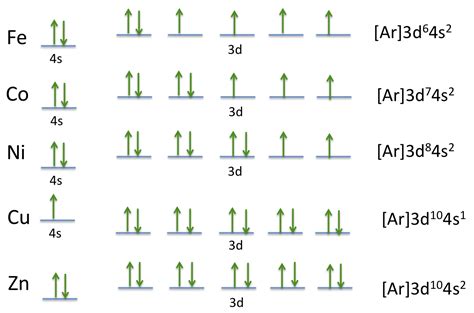
Table of Contents
What is the Electron Configuration for Cobalt? A Deep Dive into Atomic Structure
Cobalt, a transition metal with a rich history and diverse applications, holds a fascinating position in the periodic table. Understanding its electron configuration is key to unraveling its unique chemical and physical properties. This comprehensive guide will delve into the electron configuration of cobalt, explaining the underlying principles, exploring its implications, and showcasing its significance in various fields.
Understanding Electron Configuration
Before we delve into the specifics of cobalt's electron configuration, let's establish a foundational understanding of this concept. Electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. This arrangement dictates how an atom will interact with other atoms, forming chemical bonds and influencing its overall behavior. It's governed by the principles of quantum mechanics, specifically the Pauli Exclusion Principle and Hund's Rule.
-
The Pauli Exclusion Principle: This principle states that no two electrons in an atom can have the same set of four quantum numbers (principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number). This limits the number of electrons that can occupy a particular orbital.
-
Hund's Rule: This rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This maximizes the total spin of the atom, leading to a more stable configuration.
These rules are fundamental to predicting and understanding electron configurations.
Determining Cobalt's Electron Configuration
Cobalt (Co) has an atomic number of 27, meaning it possesses 27 protons and, in its neutral state, 27 electrons. To determine its electron configuration, we follow the Aufbau principle, filling orbitals in order of increasing energy. This generally follows the pattern: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, etc.
The electron configuration for cobalt is typically written as: 1s²2s²2p⁶3s²3p⁶4s²3d⁷.
Let's break this down:
- 1s²: Two electrons occupy the 1s orbital (principal quantum number n=1, azimuthal quantum number l=0).
- 2s²: Two electrons occupy the 2s orbital (n=2, l=0).
- 2p⁶: Six electrons occupy the three 2p orbitals (n=2, l=1). Each orbital holds a maximum of two electrons.
- 3s²: Two electrons occupy the 3s orbital (n=3, l=0).
- 3p⁶: Six electrons occupy the three 3p orbitals (n=3, l=1).
- 4s²: Two electrons occupy the 4s orbital (n=4, l=0). Note that the 4s orbital fills before the 3d orbital due to subtle energy differences.
- 3d⁷: Seven electrons occupy the five 3d orbitals (n=3, l=2). According to Hund's rule, these electrons will individually occupy each 3d orbital before pairing up.
The Significance of the 3d Subshell in Cobalt
The presence of seven electrons in the 3d subshell is crucial to understanding cobalt's properties. The 3d electrons are relatively loosely held, contributing to cobalt's characteristic behavior as a transition metal. This includes:
-
Variable Oxidation States: The ability of cobalt to readily lose or share electrons from its 3d and 4s orbitals results in multiple stable oxidation states, most commonly +2 and +3. This leads to a diverse range of chemical compounds.
-
Paramagnetism: The unpaired electrons in the 3d subshell make cobalt paramagnetic, meaning it is weakly attracted to magnetic fields. This property is exploited in various applications.
-
Catalysis: Cobalt's variable oxidation states and ability to form complexes make it an effective catalyst in various chemical reactions, including those involved in industrial processes and biological systems. Vitamin B12, for example, contains cobalt at its core and plays a critical role in several metabolic pathways.
-
Colorful Compounds: The 3d electrons are responsible for the characteristic colors of many cobalt compounds, due to transitions between different energy levels within the 3d subshell when interacting with light. This is often seen in pigments and dyes.
Cobalt's Role in Different Fields
Cobalt's unique properties stemming from its electron configuration have led to its widespread use in various fields:
-
Metallurgy: Cobalt is a crucial component in high-strength alloys, adding hardness, corrosion resistance, and high-temperature stability. It's used in jet engines, cutting tools, and surgical implants.
-
Magnets: Cobalt is an essential element in powerful permanent magnets, such as Alnico magnets, used in various electronic devices and motors.
-
Catalysis (Expanded): Beyond Vitamin B12, cobalt catalysts are extensively used in industrial processes like the Fischer-Tropsch process for producing synthetic fuels and in the production of various chemicals.
-
Batteries: Cobalt is a key component in lithium-ion batteries, powering a wide range of portable electronic devices and electric vehicles. However, ethical sourcing and sustainability concerns are driving research into alternative battery technologies.
-
Medicine: Besides its role in Vitamin B12, cobalt compounds are used in specific medical treatments, though their use is carefully controlled due to potential toxicity.
Beyond the Basic Configuration: Excited States and Ions
The electron configuration we've discussed, 1s²2s²2p⁶3s²3p⁶4s²3d⁷, represents cobalt in its ground state, the lowest energy configuration. However, when cobalt absorbs energy (e.g., through heating or interaction with light), electrons can be promoted to higher energy levels, resulting in excited states. These excited states have different electron configurations and can lead to different chemical and physical properties.
Similarly, cobalt ions, formed when cobalt loses electrons, will have different electron configurations compared to the neutral atom. For instance, Co²⁺ (Cobalt(II) ion) commonly has the configuration 1s²2s²2p⁶3s²3p⁶3d⁷, having lost the two 4s electrons. Co³⁺ (Cobalt(III) ion) often has the configuration 1s²2s²2p⁶3s²3p⁶3d⁶, having lost two 4s and one 3d electron. The exact configuration can be slightly more complex depending on the ligand environment.
Conclusion: The Importance of Electron Configuration
Understanding the electron configuration of cobalt, 1s²2s²2p⁶3s²3p⁶4s²3d⁷, is fundamental to comprehending its unique behavior and wide-ranging applications. The presence of seven electrons in the 3d subshell is responsible for its variable oxidation states, paramagnetism, catalytic activity, and the formation of colorful compounds. This understanding extends to cobalt's roles in metallurgy, magnets, catalysis, batteries, and medicine. While the basic configuration provides a solid foundation, remembering that excited states and ionic forms can exhibit different configurations is also crucial for a complete picture. Further exploration into the intricacies of cobalt's electronic structure will continue to unveil new insights and drive innovation in various scientific and technological domains.
Latest Posts
Latest Posts
-
Are Light Waves Transverse Or Longitudinal
Mar 22, 2025
-
What Is The Square Root Of 288
Mar 22, 2025
-
What Is 1 3 Of A Half
Mar 22, 2025
-
How Many Ounces Is 68 Grams
Mar 22, 2025
-
The Type Of Sugar Made During Photosynthesis
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Cobalt . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
