How Does Water Have A Higher Boiling Point Than Sulfide
listenit
Mar 15, 2025 · 6 min read
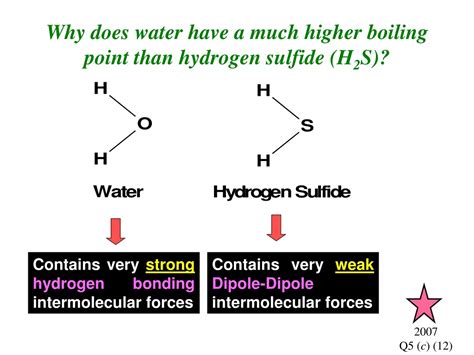
Table of Contents
How Does Water Have a Higher Boiling Point Than Hydrogen Sulfide?
Water (H₂O) and hydrogen sulfide (H₂S) are both small molecules with similar structures, yet they exhibit drastically different boiling points. Water boils at 100°C, while hydrogen sulfide boils at -60°C. This significant difference isn't merely a coincidence; it's a consequence of the unique intermolecular forces at play within each substance. Understanding this difference requires delving into the fascinating world of hydrogen bonding and van der Waals forces.
The Role of Intermolecular Forces
The boiling point of a substance is determined by the strength of the intermolecular forces holding its molecules together. When a liquid boils, the molecules gain enough kinetic energy to overcome these attractive forces and transition into the gaseous phase. Stronger intermolecular forces necessitate more energy (higher temperature) to break them, resulting in a higher boiling point.
There are several types of intermolecular forces, varying in strength:
-
London Dispersion Forces (LDFs): Present in all molecules, these forces arise from temporary fluctuations in electron distribution, creating temporary dipoles that induce dipoles in neighboring molecules. LDFs are generally weak, but their strength increases with the size and surface area of the molecule.
-
Dipole-Dipole Interactions: Occur in polar molecules (molecules with a permanent dipole moment due to differences in electronegativity between atoms). The positive end of one polar molecule attracts the negative end of another, creating a stronger attractive force than LDFs alone.
-
Hydrogen Bonding: A special type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to a lone pair of electrons on another electronegative atom in a nearby molecule. Hydrogen bonds are significantly stronger than typical dipole-dipole interactions.
Comparing Water and Hydrogen Sulfide
Both water and hydrogen sulfide are bent molecules, possessing a similar molecular geometry. However, the key difference lies in the electronegativity of the central atom and the presence of hydrogen bonding.
Hydrogen Sulfide (H₂S): Sulfur is less electronegative than oxygen. While H₂S is a polar molecule exhibiting dipole-dipole interactions, the difference in electronegativity between hydrogen and sulfur is not large enough to create strong dipole-dipole forces. The dominant intermolecular forces in H₂S are weak London Dispersion Forces. The relatively weak intermolecular forces are easily overcome at a low temperature, resulting in a low boiling point (-60°C).
Water (H₂O): Oxygen is highly electronegative. The O-H bond in water is highly polar, leading to strong dipole-dipole interactions. Crucially, water exhibits strong hydrogen bonding. Each water molecule can form up to four hydrogen bonds with neighboring water molecules, creating a vast network of interconnected molecules. This extensive hydrogen bonding network requires significantly more energy to break than the weaker intermolecular forces in H₂S. This explains the much higher boiling point of water (100°C).
The Significance of Hydrogen Bonding in Water's Properties
The exceptionally strong hydrogen bonding in water is responsible for many of its unique properties, including:
-
High boiling point: As already discussed, the strong hydrogen bonds require a substantial amount of energy to break, leading to a high boiling point.
-
High surface tension: The hydrogen bonds create a strong cohesive force between water molecules, resulting in a high surface tension. This allows water to form droplets and allows some insects to walk on water.
-
High specific heat capacity: Water can absorb a significant amount of heat without a large temperature change, due to the energy needed to break hydrogen bonds. This property is vital for regulating Earth's temperature.
-
High heat of vaporization: A considerable amount of energy is required to convert liquid water into water vapor, due to the energy needed to break hydrogen bonds. This is essential for evaporative cooling processes.
-
Density anomaly: Ice is less dense than liquid water, a unique property arising from the highly ordered hydrogen bonding network in ice, which creates a more open structure compared to liquid water.
The Impact of Molecular Weight and Polarizability
While hydrogen bonding is the dominant factor explaining the boiling point difference between water and hydrogen sulfide, it's important to acknowledge the contributions of other factors, such as molecular weight and polarizability.
Molecular Weight: H₂S (34 g/mol) has a higher molecular weight than H₂O (18 g/mol). Generally, higher molecular weight leads to stronger London Dispersion Forces. However, the effect of the increased molecular weight in H₂S is far outweighed by the strength of hydrogen bonding in water.
Polarizability: Polarizability refers to how easily the electron cloud of a molecule can be distorted. Larger and more diffuse electron clouds are more polarizable, leading to stronger LDFs. Sulfur atoms are larger and more polarizable than oxygen atoms. This contributes slightly to stronger LDFs in H₂S compared to H₂O. However, this effect is still minor compared to the impact of hydrogen bonding.
Extending the Comparison: Other Group 16 Hydrides
The trend of boiling points among the Group 16 hydrides (H₂O, H₂S, H₂Se, H₂Te) further underscores the significance of hydrogen bonding. As we move down the group, the electronegativity of the central atom decreases, and the strength of hydrogen bonding diminishes. Consequently, the boiling points of H₂S, H₂Se, and H₂Te are significantly lower than water and increase gradually with molecular weight. The decreasing electronegativity and the increasing strength of the London dispersion forces counteract the diminishing hydrogen bonding. The trend highlights the exceptional nature of water's hydrogen bonding and its influence on its boiling point.
Conclusion
The significantly higher boiling point of water compared to hydrogen sulfide is primarily attributed to the presence of strong hydrogen bonding in water, which is absent to a considerable degree in hydrogen sulfide. While molecular weight and polarizability play a role, the strength of hydrogen bonding in water far outweighs these other factors. This difference in intermolecular forces results in water possessing a unique set of properties vital for life and numerous applications. Understanding the intricate interplay of intermolecular forces is crucial for comprehending the physical and chemical behaviors of various substances. The vast network of hydrogen bonds in water makes it a truly exceptional molecule, with its properties shaping the world around us. From the weather patterns that dictate our climate to the very essence of life itself, water's properties are intrinsically linked to the remarkable strength of its hydrogen bonding. The difference in boiling points, therefore, is not just a simple observation, but a key to understanding the fundamental forces that govern the behavior of matter.
Latest Posts
Latest Posts
-
What Is The Equivalent Fraction For 5 8
Mar 15, 2025
-
How Many Seconds In 10 Years
Mar 15, 2025
-
What Is The Electron Configuration Of Krypton
Mar 15, 2025
-
The Elbow Is To The Shoulder
Mar 15, 2025
-
15 Of 80 Is What Number
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Does Water Have A Higher Boiling Point Than Sulfide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
