How Does Electronegativity Affect The Interactions Between Water Molecules
listenit
Mar 22, 2025 · 5 min read
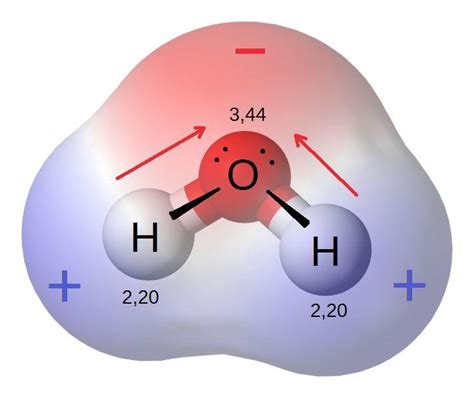
Table of Contents
How Does Electronegativity Affect the Interactions Between Water Molecules?
Water, the elixir of life, is a deceptively simple molecule with a profound impact on our world. Its unique properties, crucial for supporting life as we know it, stem directly from the interplay of its constituent atoms and the powerful force of electronegativity. This article delves deep into the relationship between electronegativity and the various interactions between water molecules, exploring the implications for water's remarkable behavior.
Understanding Electronegativity
Before diving into the intricacies of water's interactions, let's establish a firm understanding of electronegativity. Electronegativity is a measure of an atom's ability to attract shared electrons in a chemical bond. Atoms with higher electronegativity values exert a stronger pull on bonding electrons, resulting in a more polarized bond. On the Pauling scale, the most commonly used electronegativity scale, fluorine (F) holds the highest value of 4.0, while francium (Fr) has the lowest value of 0.7.
The Polarity of Water: A Consequence of Electronegativity
Water (H₂O) is a bent molecule, with a bond angle of approximately 104.5 degrees. This bent structure is crucial because it directly impacts the distribution of electron density within the molecule. Oxygen (O), with a significantly higher electronegativity than hydrogen (H) (3.44 vs. 2.20), attracts the shared electrons in the O-H bonds more strongly. This unequal sharing of electrons creates a polar covalent bond, where oxygen carries a partial negative charge (δ-) and each hydrogen carries a partial positive charge (δ+). This charge separation gives water its characteristic dipole moment, meaning it possesses a positive and negative end like a tiny magnet.
The Significance of the Dipole Moment
The dipole moment is the key to understanding the fascinating interactions between water molecules. It's the fundamental reason behind water's exceptional properties, including its high boiling point, surface tension, and ability to act as a universal solvent.
Intermolecular Forces in Water: Hydrogen Bonding
The strong electronegativity difference between oxygen and hydrogen in water leads to the formation of hydrogen bonds. These are a special type of dipole-dipole interaction, stronger than typical dipole-dipole forces but weaker than covalent bonds. A hydrogen bond occurs when a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule.
In water, the partially positive hydrogen atom of one water molecule is attracted to the partially negative oxygen atom of another water molecule. This creates a network of hydrogen bonds, holding the water molecules together and accounting for many of water's unique properties.
The Strength of Hydrogen Bonds
While weaker than covalent bonds, hydrogen bonds are remarkably strong compared to other intermolecular forces like van der Waals forces. The collective strength of numerous hydrogen bonds significantly contributes to water's high boiling point (100°C), melting point (0°C), and high specific heat capacity. Breaking these numerous hydrogen bonds requires a considerable amount of energy, explaining why water resists changes in temperature relatively well.
Hydrogen Bonding's Influence on Water's Properties
The network of hydrogen bonds explains several key properties of water:
- High boiling and melting points: The strong hydrogen bonds require significant energy to break, leading to higher boiling and melting points compared to similar-sized molecules without hydrogen bonding.
- High surface tension: The cohesive forces between water molecules due to hydrogen bonding result in a high surface tension, allowing insects to walk on water.
- High specific heat capacity: Water absorbs a large amount of heat before its temperature increases significantly, thanks to the energy needed to disrupt the hydrogen bonds. This is vital for regulating temperature on Earth.
- Excellent solvent: Water's polarity and ability to form hydrogen bonds allow it to dissolve many ionic and polar substances. The partial charges in water attract and surround ions, breaking them apart and keeping them in solution.
Other Intermolecular Forces in Water
While hydrogen bonding is the dominant intermolecular force in water, other forces also contribute to the overall interactions between water molecules:
- Dipole-dipole interactions: These weaker forces occur between the partially positive and negative ends of water molecules. While less significant than hydrogen bonding, they still contribute to the overall cohesion of water.
- London Dispersion Forces (LDFs): Even nonpolar molecules experience fleeting, temporary dipoles due to the random movement of electrons. These induce dipoles in neighboring molecules, leading to weak London Dispersion Forces. While the weakest of the intermolecular forces, LDFs exist between all molecules, including water molecules.
The Impact of Electronegativity on Water's Behavior
The overarching theme here is that the substantial electronegativity difference between oxygen and hydrogen is the driving force behind water's remarkable properties. Without this difference, water would not be polar, hydrogen bonds wouldn't form, and the properties crucial for supporting life wouldn't exist.
Electronegativity and Solvent Properties
Water's excellent solvent properties are directly linked to its electronegativity. The highly electronegative oxygen atom attracts positive ions, while the less electronegative hydrogen atoms attract negative ions. This ability to interact with both positively and negatively charged ions allows water to dissolve a wide range of substances, making it essential for biological processes and chemical reactions.
Electronegativity and Biological Significance
The unique properties of water stemming from its electronegativity are fundamental for life:
- Biological solvent: Water's solvent properties allow for the transport of nutrients and the removal of waste products within living organisms.
- Temperature regulation: Water's high specific heat capacity helps maintain stable temperatures in living organisms and environments.
- Structural role: Water plays a crucial structural role in biological molecules like proteins and nucleic acids, influencing their shape and function.
- Chemical reactions: Water participates in countless chemical reactions within living systems, acting as a reactant or solvent.
Conclusion: Electronegativity and the Water Molecule
The electronegativity difference between oxygen and hydrogen is the linchpin in understanding the behavior of water molecules. This difference creates the polar nature of water, leading to the formation of strong hydrogen bonds and other intermolecular interactions. These interactions, in turn, give rise to water's unique properties, making it essential for life on Earth and shaping the physical and chemical world around us. Understanding electronegativity’s role in shaping water's properties is crucial across diverse scientific disciplines, from biology and chemistry to geology and atmospheric science. The intricate dance of electrons, governed by electronegativity, makes water far more than just a simple molecule; it's a fundamental force shaping our planet and the life it sustains.
Latest Posts
Latest Posts
-
Heat Of Neutralization For Hcl And Naoh
Mar 24, 2025
-
What Type Of Bond Holds Amino Acids Together
Mar 24, 2025
-
Organism That Cannot Make Its Own Food
Mar 24, 2025
-
How Long Would It Take To Get To Andromeda
Mar 24, 2025
-
What Is The Square Root Of 175
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How Does Electronegativity Affect The Interactions Between Water Molecules . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
