How Does Atomic Radius Change Across A Period
listenit
Mar 22, 2025 · 5 min read
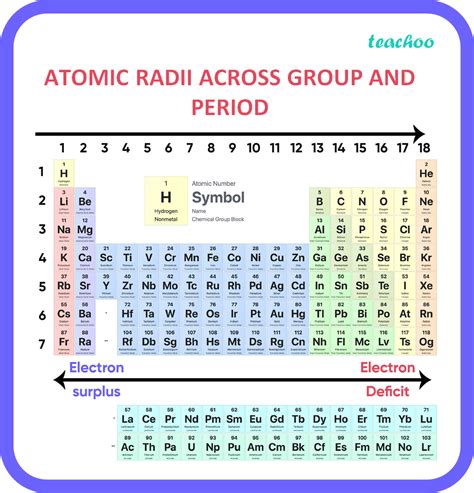
Table of Contents
How Does Atomic Radius Change Across a Period? A Comprehensive Guide
Understanding the periodic trends of elements is fundamental to grasping the principles of chemistry. One such crucial trend is the change in atomic radius across a period (row) of the periodic table. This article delves deep into this phenomenon, explaining the underlying reasons, exceptions, and its implications in various chemical and physical properties.
What is Atomic Radius?
Before we explore the changes, let's define atomic radius. Atomic radius refers to the distance from the nucleus to the outermost stable electron orbital of an atom. It's crucial to understand that this isn't a hard boundary like a solid sphere; it's more of a probabilistic distribution representing the likely location of the valence electrons. Measuring it directly is challenging, so we often use various methods, including covalent radius (half the distance between two bonded atoms), metallic radius (half the distance between two adjacent atoms in a metallic lattice), and van der Waals radius (half the distance between two non-bonded atoms). For the sake of simplicity and consistency, we'll predominantly focus on the general trend observed across a period.
The Trend: Decrease Across a Period
The overarching trend is a decrease in atomic radius as you move from left to right across a period. This seemingly simple statement hides a complex interplay of fundamental forces within the atom. Let's dissect this observation.
The Role of Nuclear Charge
As you progress across a period, the number of protons in the nucleus increases. This increase in positive charge strongly attracts the electrons, pulling them closer to the nucleus. This increased electrostatic attraction is the primary driver behind the decrease in atomic radius.
Shielding Effect (or Screening Effect)
While the nuclear charge increases, the number of electron shells remains constant across a period. Electrons in inner shells partially shield the outermost electrons (valence electrons) from the full effect of the nuclear charge. This shielding effect is not perfect; the valence electrons still experience a significant portion of the nuclear charge. However, the increase in nuclear charge outweighs the increase in shielding, leading to a net increase in the effective nuclear charge experienced by the valence electrons. This further contributes to the reduction in atomic radius.
Effective Nuclear Charge
Effective nuclear charge (Z<sub>eff</sub>) is a crucial concept here. It represents the net positive charge experienced by an electron, considering the shielding effect of other electrons. A higher Z<sub>eff</sub> implies a stronger pull on the valence electrons, thus decreasing the atomic radius. Across a period, Z<sub>eff</sub> increases, leading to the observed shrinking of atomic size.
Exceptions and Subtleties
While the general trend is a decrease in atomic radius across a period, some exceptions and subtleties exist:
-
Transition Metals: The decrease in atomic radius across the transition metal series is less pronounced than in other periods. This is because the added electrons are filling the inner d orbitals, which don't significantly increase the shielding effect compared to the increase in nuclear charge. The effect of increasing nuclear charge is dominant but less dramatic than in other periods.
-
Lanthanide and Actinide Contraction: The lanthanides and actinides experience a particularly significant contraction in atomic radius due to the poor shielding effect of the f-orbitals. This contraction has knock-on effects on the properties of subsequent elements.
Implications of Atomic Radius Change
The change in atomic radius across a period has far-reaching implications:
Ionization Energy
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. This is directly linked to the decrease in atomic radius. As the valence electrons are held more tightly by the increased effective nuclear charge, more energy is required to remove them.
Electronegativity
Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period. A smaller atom with a higher effective nuclear charge can more effectively attract shared electrons in a covalent bond.
Metallic Character
Metallic character, the tendency of an element to lose electrons and form positive ions, generally decreases across a period. The stronger attraction of the nucleus to the valence electrons makes it harder for them to be lost, reducing the metallic character.
Chemical Reactivity
The change in atomic radius directly influences the chemical reactivity of elements. Elements on the left side of a period tend to be more reactive, readily losing electrons to achieve a stable electron configuration. Elements on the right side are generally less reactive, with the noble gases being exceptionally unreactive due to their filled valence shells.
Visualizing the Trend: A Periodic Table Perspective
Imagine the periodic table. As you move from left to right across a period, visualize the atoms shrinking in size. This visual representation helps solidify the understanding of the trend. Consider the alkali metals (Group 1) with their relatively large atomic radii compared to the halogens (Group 17) with their smaller radii within the same period.
Conclusion: A Fundamental Trend with Broad Implications
The decrease in atomic radius across a period is a fundamental periodic trend with far-reaching consequences. It is not merely an isolated observation; it underpins many other periodic trends, including ionization energy, electronegativity, and metallic character. Understanding this trend provides a critical foundation for comprehending the behavior and properties of elements and their interactions, forming the bedrock of chemical understanding. This interplay of nuclear charge, shielding, and effective nuclear charge provides a comprehensive explanation for this vital periodic property. The exceptions and subtle variations observed only serve to highlight the complexity and richness of the chemical world, making the study of atomic radius a fascinating and important pursuit in chemistry.
Latest Posts
Latest Posts
-
What Is The Number Of Protons In Potassium
Mar 23, 2025
-
How Is Power And Work Related
Mar 23, 2025
-
How To Put Eulers Number In Excel
Mar 23, 2025
-
Quadrilateral Two Pairs Of Parallel Sides
Mar 23, 2025
-
What Is The Lcm For 3 And 8
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Does Atomic Radius Change Across A Period . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
