What Is The Number Of Protons In Potassium
listenit
Mar 23, 2025 · 6 min read
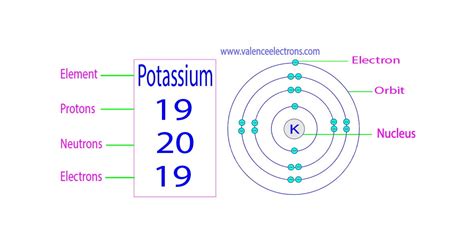
Table of Contents
What is the Number of Protons in Potassium? A Deep Dive into Atomic Structure
Potassium, a vital element for life, plays a crucial role in various biological processes. Understanding its atomic structure, particularly the number of protons it possesses, is fundamental to comprehending its chemical behavior and biological significance. This article delves deep into the answer to the question: What is the number of protons in potassium? and explores related concepts in atomic physics and chemistry.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we pinpoint the number of protons in potassium, let's review the fundamental building blocks of an atom. Every atom consists of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element; it's the element's atomic number.
- Neutrons: Neutrally charged particles also located in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. They are much lighter than protons and neutrons.
The number of protons dictates an atom's identity. Changing the number of protons fundamentally alters the element itself. Adding or removing neutrons creates isotopes of the same element (atoms with the same number of protons but a different number of neutrons). Altering the number of electrons results in ions (atoms with a net positive or negative charge due to an imbalance of protons and electrons).
The Atomic Number of Potassium: Unveiling the Number of Protons
The key to determining the number of protons in potassium lies in its atomic number. The atomic number is a unique identifier for each element, representing the number of protons in the nucleus of an atom of that element. This number is typically represented by the symbol 'Z'.
Potassium's atomic number is 19. This fundamental fact unequivocally answers our initial question: Potassium has 19 protons. This consistent number of protons distinguishes potassium from all other elements on the periodic table.
Isotopes of Potassium: Variations in Neutron Count
While the number of protons remains constant at 19 for all potassium atoms, the number of neutrons can vary. These variations result in different isotopes of potassium. Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. The mass number (A) of an isotope is the sum of its protons and neutrons.
Naturally occurring potassium consists of three isotopes:
- Potassium-39 (³⁹K): This is the most abundant isotope, comprising about 93.3% of natural potassium. It has 19 protons and 20 neutrons (19 + 20 = 39).
- Potassium-40 (⁴⁰K): This isotope is radioactive, making up about 0.012% of natural potassium. It has 19 protons and 21 neutrons (19 + 21 = 40). It decays through beta decay and electron capture.
- Potassium-41 (⁴¹K): This stable isotope accounts for approximately 6.7% of natural potassium. It contains 19 protons and 22 neutrons (19 + 22 = 41).
The presence of the radioactive isotope, potassium-40, is significant in various fields, including geological dating and medical imaging. The radioactive decay of ⁴⁰K is used in radiometric dating techniques to determine the age of rocks and minerals.
The Significance of Potassium's 19 Protons in its Chemical Behavior
Potassium's 19 protons significantly influence its chemical properties and reactivity. The arrangement of electrons in its electron shells determines how it interacts with other atoms. Potassium has one valence electron in its outermost shell, making it highly reactive and prone to losing this electron to achieve a stable electron configuration.
This tendency to lose one electron results in the formation of a +1 ion (K⁺). This ion is essential for various biological processes, including:
- Maintaining fluid balance: Potassium ions regulate the distribution of water and electrolytes within the body.
- Nerve impulse transmission: Potassium ions play a vital role in the transmission of nerve impulses.
- Muscle contraction: Potassium ions are crucial for muscle contraction and relaxation.
- Enzyme activation: Potassium acts as a cofactor for many enzymes, helping them function properly.
- Blood pressure regulation: Potassium levels influence blood pressure.
The loss of a single electron, driven by the presence of 19 protons, underpins potassium's crucial biological role.
Potassium's Role in Biology: A Closer Look
The biological significance of potassium stems directly from its chemical properties, which are, in turn, determined by its 19 protons. Potassium's presence is ubiquitous in living organisms, where it participates in diverse processes:
Potassium Channels: Gatekeepers of Cellular Processes
Potassium channels are transmembrane proteins that facilitate the selective passage of potassium ions across cell membranes. These channels are crucial for regulating various cellular processes, including:
- Action potentials in neurons: The controlled movement of potassium ions across neuronal membranes generates action potentials, the electrical signals that transmit information within the nervous system.
- Heart rhythm: The rhythmic flow of potassium ions through heart muscle cells is essential for maintaining a regular heartbeat.
- Cell volume regulation: Potassium channels contribute to maintaining the appropriate volume of cells by controlling the movement of water and ions.
The precise control of potassium ion flow, enabled by the properties of potassium ions dictated by its 19 protons, is crucial for the proper functioning of these critical cellular processes.
Potassium in Plant Physiology: Essential for Growth and Development
In plants, potassium plays a vital role in various physiological processes, including:
- Stomatal regulation: Potassium ions are involved in the opening and closing of stomata, the tiny pores on plant leaves that regulate gas exchange and water loss.
- Enzyme activation: Similar to animals, potassium acts as a cofactor for several plant enzymes, influencing metabolic processes.
- Nutrient uptake: Potassium facilitates the uptake of other essential nutrients by plant roots.
- Disease resistance: Adequate potassium levels enhance plant resistance to diseases and stress.
These crucial roles highlight the profound impact of potassium's 19 protons on plant life and the overall health of the ecosystem.
Potassium Deficiency and Excess: Health Implications
Maintaining appropriate potassium levels is critical for health. Both deficiency and excess can lead to serious health problems:
Potassium Deficiency (Hypokalemia): This can result from inadequate dietary intake, excessive fluid loss (e.g., vomiting, diarrhea), or certain medical conditions. Symptoms can include muscle weakness, fatigue, heart irregularities, and digestive problems. Severe hypokalemia can be life-threatening.
Potassium Excess (Hyperkalemia): This can occur due to kidney problems, certain medications, or excessive potassium intake. Symptoms can include muscle weakness, heart problems (including potentially fatal arrhythmias), and nausea.
The importance of maintaining proper potassium balance underscores the vital role of this element in overall health and well-being, a role directly linked to its atomic structure and the presence of 19 protons.
Conclusion: The Significance of 19 Protons
The simple answer to "What is the number of protons in potassium?" is 19. However, this seemingly simple number holds immense significance. It defines potassium as a unique element, dictates its chemical properties, and fundamentally influences its crucial biological roles in both plants and animals. Understanding the number of protons in potassium is not just a matter of atomic physics; it is fundamental to comprehending the intricate mechanisms of life itself. From regulating nerve impulses to maintaining the rhythm of the heart and ensuring the health of plants, the 19 protons in potassium are vital for a healthy and functioning world. Further research continues to unveil the multifaceted roles of potassium, further emphasizing the importance of this seemingly simple number in the vast world of chemistry and biology.
Latest Posts
Latest Posts
-
What Is A Coefficient In Chemical Equations
Mar 25, 2025
-
1 Is What Percent Of 200
Mar 25, 2025
-
8 Is What Percent Of 25
Mar 25, 2025
-
Quadrilateral With 2 Pairs Of Parallel Sides
Mar 25, 2025
-
1 3 Divided By 4 In Fraction
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Number Of Protons In Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
