Horizontal Rows Of The Periodic Table Are Called
listenit
Mar 17, 2025 · 7 min read
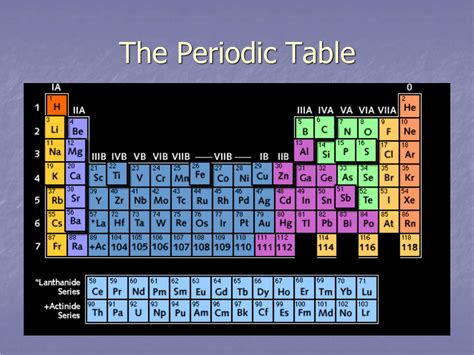
Table of Contents
Horizontal Rows of the Periodic Table are Called: Periods – A Deep Dive into Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. Understanding its structure is crucial for grasping the behavior of elements and predicting their reactions. One fundamental aspect of this structure is the arrangement of elements into horizontal rows, known as periods. This article delves deep into what periods represent, the trends observed within them, and their significance in chemistry.
What are Periods in the Periodic Table?
The horizontal rows in the periodic table are called periods. Each period corresponds to a principal energy level (or shell) that is being filled with electrons. As we move across a period from left to right, the atomic number increases, signifying an addition of a proton and, generally, an electron. This addition of electrons influences the element's electronic configuration and, consequently, its chemical properties.
The number of elements in each period varies because the number of orbitals available at each principal energy level differs. This directly impacts the number of electrons that can occupy those energy levels and therefore, the number of elements within a given period.
-
Period 1 (n=1): Contains only two elements, hydrogen (H) and helium (He), because the first principal energy level (n=1) only has one subshell, the 1s subshell, which can hold a maximum of two electrons.
-
Period 2 (n=2): Has eight elements, from lithium (Li) to neon (Ne). This is because the second principal energy level (n=2) has two subshells: 2s and 2p, with a total capacity of eight electrons.
-
Period 3 (n=3): Also contains eight elements, from sodium (Na) to argon (Ar). While the third principal energy level (n=3) has three subshells (3s, 3p, and 3d), only the 3s and 3p subshells are filled in this period. The 3d subshell is filled later.
-
Period 4 (n=4) onwards: The pattern becomes more complex as the number of subshells and the capacity for electrons increases, leading to longer periods with varying numbers of elements.
Periodic Trends within Periods: A Graded Explanation
Moving across a period reveals predictable trends in various atomic properties. These trends are crucial for understanding reactivity and other chemical behaviors.
1. Atomic Radius: Decreasing Across a Period
Atomic radius refers to the size of an atom. Across a period, the atomic radius generally decreases. This is because, as we add protons to the nucleus, the positive charge increases, attracting the electrons more strongly. While additional electrons are also being added, they are going into the same principal energy level, not expanding the atom significantly. The increased nuclear charge outweighs the electron shielding effect, pulling the electrons closer to the nucleus and resulting in a smaller atomic radius.
2. Ionization Energy: Increasing Across a Period
Ionization energy is the energy required to remove an electron from a neutral atom. Across a period, ionization energy generally increases. This is a direct consequence of the decreasing atomic radius. As the electrons are held more tightly by the nucleus due to the increased nuclear charge, more energy is required to remove them.
3. Electronegativity: Increasing Across a Period
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Across a period, electronegativity generally increases. This is closely linked to ionization energy and atomic radius. Atoms with smaller atomic radii and higher ionization energies tend to attract electrons more strongly in a bond.
4. Electron Affinity: Generally Increases Across a Period (with exceptions)
Electron affinity is the energy change that occurs when an electron is added to a neutral atom. While a general trend is an increase across a period, there are notable exceptions. The addition of an electron is usually exothermic (releases energy), but factors like electron-electron repulsion in already occupied subshells can affect this trend, leading to deviations.
5. Metallic Character: Decreases Across a Period
Metallic character refers to the properties associated with metals, such as conductivity, malleability, and ductility. Across a period, metallic character generally decreases. Metals are typically found on the left side of the periodic table, while nonmetals are on the right. As we move from left to right, the elements become less metallic and more non-metallic in their properties. This change is related to the increasing ionization energy and electronegativity.
The Significance of Periods in Predicting Chemical Behavior
The periodic trends within periods are not just theoretical observations; they have practical applications in predicting the chemical behavior of elements.
-
Reactivity: The position of an element within a period gives insights into its reactivity. Elements on the far left (alkali metals) are highly reactive due to their low ionization energies, while elements on the far right (noble gases) are extremely unreactive due to their stable electron configurations.
-
Bonding: The electronegativity values help predict the type of bonds that an element will form. Elements with large electronegativity differences form ionic bonds, while elements with similar electronegativities form covalent bonds.
-
Oxidation States: The position within a period helps predict the possible oxidation states that an element can exhibit.
-
Compound Formation: The trends in properties allow chemists to anticipate the type of compounds that an element is likely to form, their stability, and their properties.
Beyond the Basic Trends: A Deeper Look at Periodicity
While the trends described above represent general tendencies, it’s important to acknowledge that exceptions exist. The complexity of electron-electron interactions and other subtle quantum mechanical effects can lead to deviations from these ideal trends. For example, the electron affinity of some elements does not follow the strictly increasing trend across a period. The d-block and f-block elements exhibit more nuanced periodic trends because of the complex interactions between their electrons.
Periods and the Filling of Subshells: A Detailed Analysis
The arrangement of elements within periods is directly related to the filling of electron subshells. The Aufbau principle guides the order in which electrons fill these subshells. This principle is fundamental to understanding the electronic configurations of atoms and their properties. Let's revisit the significance of subshells in shaping the periods:
-
s-block elements: The first two elements of each period (except for period 1) belong to the s-block, where the s subshell is being filled. These elements generally exhibit metallic character and have relatively low ionization energies.
-
p-block elements: The last six elements of each period (from period 2 onwards) belong to the p-block, where the p subshell is being filled. This block features a wide range of elements, including both metals and nonmetals, reflecting the diversity in their chemical properties.
-
d-block elements (transition metals): These elements occupy periods 4-7 and are characterized by the filling of the d subshell. Transition metals exhibit a variety of oxidation states and often form colorful compounds due to their ability to readily lose electrons from the d subshell.
-
f-block elements (inner transition metals): These elements, including the lanthanides and actinides, are characterized by the filling of the f subshell. These elements primarily occur in the lower periods and are known for their complex chemistry.
Applications of Periodicity: From Basic Chemistry to Advanced Materials Science
Understanding the periodic trends and the structure of periods isn't simply an academic exercise; it has extensive practical applications across various scientific fields:
-
Material Science: The properties of elements within periods are carefully considered when designing new materials with specific functionalities. For example, understanding the trends in conductivity and reactivity is critical when developing new semiconductors or superconductors.
-
Catalysis: The ability of transition metals to exhibit variable oxidation states makes them crucial as catalysts in numerous chemical reactions, including many industrial processes. The understanding of periodic trends helps in the selection and optimization of catalysts.
-
Medicine: The properties of elements, governed by their positions within periods, are exploited in the development of various medicines and medical technologies. For instance, understanding the biocompatibility and reactivity of specific elements is crucial in designing medical implants and drug delivery systems.
-
Environmental Science: The behavior of elements in the environment is intimately tied to their periodic properties. Understanding how elements react with water, soil, and air is essential for environmental remediation and pollution control.
Conclusion: Periods – The Backbone of Chemical Understanding
The horizontal rows of the periodic table, known as periods, are fundamental to understanding the organization and behavior of elements. The trends in atomic properties within periods are not merely abstract concepts; they provide a powerful framework for predicting and explaining chemical phenomena across a vast range of applications. From predicting the reactivity of an element to designing new materials, a solid understanding of periods is an essential foundation for success in chemistry and related scientific disciplines. The continuous exploration and refinement of our understanding of periodic trends promise even more innovative applications in the future, cementing the enduring importance of this fundamental concept.
Latest Posts
Latest Posts
-
1 2 A Pound Is How Many Ounces
Mar 17, 2025
-
An Allele That Masks Another Allele Is Known As
Mar 17, 2025
-
Equal To The Number Of Protons
Mar 17, 2025
-
How Many Degrees Celsius Is 1 Degrees Fahrenheit
Mar 17, 2025
-
How Do You Find Heat Energy That Water Gains
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Horizontal Rows Of The Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
